

The following Guideline provides general guidance in relation to Health Risk Assessment Methodology in the assessment of site contamination. This Guideline forms part of the National Environment Protection (Assessment of Site Contamination) Measure 1999 and should be read in conjunction with that document, which includes a Policy Framework and Assessment of Site Contamination flowchart. The National Environment Protection Council acknowledges the contribution of the National Health and Medical Research Council to the development of this Measure.
|
Contents Health Risk Assessment Methodology
| ||
|
|
|
|
| Page |
Summary...................................................................... | 1 | |
Abbreviations................................................................... | 4 | |
Glossaries...................................................................... | 5 | |
| 5 | |
| 3.2 General terms | 8 |
Framework for Risk Assessment...................................................... | 14 | |
Data Collection.................................................................. | 18 | |
| 18 | |
Exposure Assessment.............................................................. | 19 | |
| 19 | |
| 6.2 Key Points in Exposure Assessments of Contaminated Sites | 19 |
| 6.3 Use of Point Estimates and Probability Distributions | 20 |
| 26 | |
| 6.5 Exposure Durations and Exceedances of Acceptable Daily Intakes (ADIs) | 27 |
| 27 | |
| 6.7 Choice of a Test | 28 |
| 30 | |
| 31 | |
| 31 | |
| 33 | |
| 34 | |
| 34 | |
| 6.14 Default Values for Exposure Assessments | 35 |
| 6.15 Sources of Exposure Assessment Data | 36 |
7. | Toxicity Assessment............................................................... | 36 |
| 7.1 Introduction | 36 |
| 38 | |
| 41 | |
| 41 | |
| 7.5 Methods for Risk Assessment of Carcinogens | 42 |
Risk Characterisation............................................................... | 49 | |
| 49 | |
| 49 | |
| 8.3 Conceptual Guide for Developing Chemical-Specific Risk Characterisations | 51 |
| 51 | |
| 52 | |
Appraisal of Assessments............................................................ | 55 | |
| 55 | |
| 55 | |
| 57 | |
10. | Use of Health Risk Assessment to develop health-based soil criteria............................... | 59 |
References....................................................................... | 63 |
1. SUMMARY
Site-specific health risk assessment provides an appraisal of the nature and magnitude of the risks arising from chemical contamination of a site. The assessment takes into account factors relevant to the site such as the proposed use, physico-chemical and bioavailability characteristics of the particular contaminant(s), and the depth and distribution of the contamination. Health risk assessment complements the process of ecological risk assessment.
Site-specific health risk assessment is intended "to provide complete information to risk managers, specifically policymakers and regulators, so that the best possible decisions are made" (Paustenbach, 1989, p28). Good risk assessment is dependent upon a high degree of objectivity and scientific skill and should be distinguished from the risk management process which selects options in response to health risk assessments and which incorporates "scientific, social, economic and political information" and which "requires value judgements eg on the tolerability of risk and reasonableness of costs" (ANZECC/NHMRC 1992, piii).
A preliminary site-specific appraisal risk assessment can be undertaken by comparing site results with the Health-based Investigation Levels appropriate to the site and to its current or proposed use. These are derived using risk assessment techniques and can be applied generically to a range of exposure settings. Where there are exceedances of the Health-based Investigation Levels, site-specific health risk assessments may be used to determine whether further action is needed for a site. The action may range from informing residents or owners of the site of the contamination, to requiring large scale remediation.
The process of risk assessment is intended to achieve the following objectives when assessing site contamination (US EPA 1989):
• to establish baseline risks and whether site remediation or other action is necessary;
• to determine a tolerable level of contaminants that can remain in place with adequate protection of public health;
• to enable comparison of potential health impacts of various remediation techniques; and
• to provide a consistent method of appraising and recording public health risks at sites.
There are several models of risk assessments and various sets of definitions for the relevant terms. This document uses a model comprising:
• data collection and evaluation of the chemical condition of the site;
• toxicity assessment of contaminants;
• exposure assessment for the population on or near the site; and
• risk characterisation (US EPA 1989).
These four stages are closely linked and highly dependent on the preceding stages.
Data collection entails the acquisition and analysis of information about chemicals on a site that may affect human health and which will be the focus for the particular risk assessment (US EPA, 1989).
Toxicity assessment considers:
• the nature of adverse effects related to the exposure;
• the dose-response relationship for various effects;
• the weight of evidence for effects such as carcinogenicity; and
• the relevance of animal data to humans.
Both qualitative and quantitative toxicity information is evaluated to determine "the incidence of adverse effects occurring in humans at different exposure levels" (US EPA, 1989, p 1.6).
Exposure assessment involves the determination of the frequency, extent and duration of exposures in the past, currently, and in the future. There is also the identification of exposed populations and particularly sensitive subpopulations, and potential exposure pathways. Environmental monitoring and predictive models can be used to determine the levels of exposure at particular points on the exposure pathways. The contaminant intakes from the various pathways under a range of scenarios can then be estimated (US EPA, 1989).
Given this information, risk characterisation details the nature and potential incidence of effects for the exposure conditions described in the exposure assessment. An integral part of this stage is to evaluate the uncertainties and assumptions in the risk assessment process (Langley and El Saadi, 1991). The uncertainties should be "taken into account in planning the management of a site" (ANZECC/NHMRC 1992, p34). The uncertainties may be addressed by gathering further information, the incorporation of safety factors (eg in the development of criteria) and conservatism, and professional judgement.
"The process of risk assessment should enable consistent decisions to be made by the specialists undertaking the process. Expert professional judgement is an integral part of the process. Site-specific risk assessments should not lead to significant variations in the management of similar sites" (ibid, p34)
In many instances site-specific health risk assessments will not be necessary as problems will be 'obvious' and the significant resources required for an adequate site-specific risk assessment or the generation of site-specific soil criteria should be directed to the management of the site. For some sites health risk assessment may be unnecessary as "there may be no population at risk, or decisions may be made on other grounds" (ibid, p20). Site-specific risk assessments may be required as part of the Occupational Health and Safety procedures relevant to site assessment activities.
Numerical estimates of risk will rarely be feasible because of "limitations in toxicological and exposure data" (ibid, p34) which will be reflected in the uncertainty assessment, but a degree of quantification may be possible for some components such as data collection and exposure assessment.
It should be recognised that, as a consequence of data limitations (for example, from the rates of sampling and the analytes chosen), site-specific health risk assessment is a screening process where there may be low rates of false negatives and false positives. "Risk assessment is based on probabilities rather than absolutes and this should be reflected in decision-making" (ibid, p34).
This document provides an approach to site-specific health risk assessment. Due to the complexity and scale of the health risk assessment process a concise 'cookbook' is not practicable. Similarly, the site-specific issues are often sufficiently complex and 'site-specific' for a particular site that a manageable and complete algorithm for decision-making cannot be drafted: the document provides a series of guidelines (and prescriptions) to assist the decision-making process. Where possible, the document is prescriptive about certain aspects of risk assessment. Having specific requirements for the content of investigations and having them presented in uniform, coherent and logically developed reports will enable more efficient, accurate, timely and transparent decision-making and a greater consistency of decision-making across Australia. The principles and guidelines in this document are intended to assist that process and the qualitative process of determining whether remediation is required or not for the proposed use.
The site-specific process is a multi-disciplinary task and requires considerable expertise. People involved in specific components of the health risk assessment process should be adequately qualified and experienced and have a broad understanding of health risk assessment and management and the practical realities of contaminated sites. Professional skills that may be used include: soil science, engineering, geology, history, chemistry, planning, statistics, occupational hygiene, occupational and public health medicine, environmental health, toxicology and health science and epidemiology. While it is unlikely that one person will have the breadth of skill to undertake all components of the health risk assessment, there must be a single person coordinating and taking responsibility for the assessment.
2. ABBREVIATIONS
ADI | Acceptable Daily Intake (WHO) |
ANZECC | Australia and New Zealand Environment and Conservation Council |
ASCEPT | Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists |
BMD | Benchmark Dose |
BMDL | Lower confidence limit on BMD |
BMR | Benchmark Risk (Response) |
DOH | Department of Health (United Kingdom) |
DNA | Deoxyribonucleic acid |
ECETOC | European Centre for Ecotoxicology and Toxicology of Chemicals |
FDA | Food and Drug Administration (USA) |
GCP | Good Clinical Practice |
GLP | Good Laboratory Practice |
IARC | International Agency for Research on Cancer |
IPCS | International Programme on Chemical Safety |
IRIS | Integrated Risk Information System |
JECFA | Joint FAO/WHO Expert Committee on Food Additives |
JMPR | Joint FAO/WHO Meeting on Pesticide Residues |
LED | Lowest Effective Dose |
LOAEL | Lowest Observed Adverse Effect Level |
MTD | Maximum Tolerated Dose |
NEPC | National Environment Protection Council (Australia) |
NHMRC | National Health and Medical Research Council (Australia) |
NICNAS | National Industrial Chemical Notification and Assessment Scheme |
NOAEL | No Observed Adverse Effect Level |
NOHSC | National Occupational Health and Safety Commission |
NTP | National Toxicology Program (USA) |
OECD | Organisation for Economic Cooperation and Development |
PCB | Polychlorinated biphenyl |
PTWI | Provisional Tolerable Weekly Intake (WHO) |
q1* | The 95% upper confidence limit of the slope estimate used for the linear multi-stage model |
QRA | Quantitative Risk Assessment |
RfD | Reference Dose (US EPA) |
SF | Safety Factors |
SAR | Structure Activity Relationship |
TDI | Tolerable Daily Intake (WHO) |
TWP | Technical Working Party |
US EPA | United States Environmental Protection agency |
WHO | World Health Organization |
3. GLOSSARIES
3.1 Soil Criteria Terms
There are two prerequisites for comparison of soil and water test results with defined criteria. The first prerequisite is a standardised soil sampling methodology which provides an appropriate amount of information about the distribution and level of contaminants on a piece of land. The second is a standardised approach to data analysis to enable a meaningful interpretation of sampling results.
3.1.1 Investigation Levels:
An investigation level is the concentration of a contaminant above which further appropriate investigation and evaluation will be required. The investigation and evaluation is to ascertain:
• the typical and extreme concentrations of the contaminant(s) on the site;
• the horizontal and vertical distribution(s) of the contaminant(s) on the site;
• the physico-chemical form(s) of the contaminant(s);
• the bioavailability of the contaminant(s).
(Langley and El Saadi 1991)
Health-based Soil Investigation Levels are not intended to be clean up levels.
Levels slightly in excess of the investigation levels do not imply unacceptability or levels likely to pose a significant health risk (See Figure 3-I).
Once the further investigation(s) is (are) completed, a site-specific health risk assessment will be required to determine the presence of health risk and, if present, its nature and degree. Final assessment of the degree of contamination should take into account any uncertainties arising from the sampling and analytical methodologies.
When dealing with substances which are considered to have possible effects at very low doses (eg. some carcinogens), a specific approach will need to be established to derive the investigation and response levels. The NHMRC Technical Working Party
on the Carcinogenic Risk Assessment for Soil Contaminants has established Guidelines for Cancer Risk Assessment (NHMRC, 1999).
The application of Investigation Levels and Response Levels to site management will be guided by the risk management process which will be driven by scientific, technological, social, political and economic factors.
Investigation levels provide a trigger to assist in judging whether a detailed investigation of a site is necessary.
When assessing the environmental/human health significance of levels of contamination above an investigation level, the following factors should be considered: potential ground water contamination; land use; the history and nature of the contamination; evidence of potential contamination from site inspection; the local background levels; the problems of the presence of multiple contaminants; and the size of the site. Exposure pathways will be more diverse for a larger site.
The principal limitations of health-based soil investigation levels are that they:
• do not apply to land being, or proposed to be, used for agricultural and forestry purposes (consult the relevant agricultural and forestry departments for the appropriate criteria for agricultural land.)
• do not take into account all environmental concerns (for example, the potential effects on wildlife): where relevant, these would require further consideration. (adapted from EPA NSW, 1997)
3.1.2 Response Levels:
Response Levels are the concentrations of contaminants at a specific site assessment for which some form of response is required to provide an adequate margin of safety to protect public health and/or the environment.
(adapted from Langley and El Saadi 1991)
Different Response Levels are intended to be used for different exposure situations (eg. residential, recreational, or commercial/industrial land uses).
Figure 3-I
The relationship of soil criteria levels for Substance X.

Proposed Land Uses:
1. Residential
2. Recreational
3. Residential (minimal exposure)
4. Commercial/Industrial
(Figure not to scale, sequence of '1234' will vary from substance to substance. For example, for another substance, the sequence may be 2134).
(adapted from ANZECC/NHMRC, 1992, p36)
Site-specific evaluation of the available data and proposed land use will be required to determine whether single, occasional or typical values in excess of the investigation level will prompt the further investigation.
Overt health effects would not be expected to occur until contamination is present at levels well in excess of response levels.
The nature of the response required to protect human health will depend on the assessment of risk associated with a given level of contamination. Where the risk is assessed as being relatively low, the response may simply involve informing occupants of the site so that they are aware of risks arising from, certain activities such as, pica behaviour in children (see Schedule B(7A), section 12.2). In cases where there is a relatively high risk, complex soil treatment may be required.
More specifically, the nature of the response will be modulated by factors including:
• Land use eg. residential, recreational or commercial/ industrial.
• Potential child occupancy.
• Potential environmental effects including leaching into groundwater.
• Single or multiple contaminants.
• Depth of contamination.
• Level and distribution of contamination.
• Bioavailability of the contaminant(s) eg. related to speciation, route of exposure.
• Toxicological assessment of the contaminant(s) eg. toxicokinetics, carcinogenicity, acute and chronic toxicity.
• Physico-chemical properties of the contaminant(s).
• State of the site surface eg. paved, grassed or exposed.
• Potential exposure pathways.
• Uncertainties with the sampling methodology and toxicological assessment.
Where a site specific assessment is being carried out with a view to defining response levels, consideration should also be given to the possible risk associated with mixtures of contaminants, since in some circumstances such risks may necessitate a more or less extensive response than would be required to deal with a single contaminant." (Langley and El Saadi 1991, Imray and Langley 1996)
3.2 General terms
(Adapted from NHMRC 1997)
ADI is the Acceptable Daily Intake. The daily intake of a chemical which, during a lifetime, appears to be without appreciable risk, on the basis of all the facts known at the time. It is expressed in milligrams per kilogram of body weight per day (mg/kg/day) (WHO, 1989a)
Adverse Effect is the change in morphology, physiology, growth, development or life span of an organism which results in impairment of functional capacity or impairment of capacity to compensate for additional stress or increase in susceptibility to the harmful effects of other environmental influences.
Adduct is a chemical moiety which is covalently bound to a large molecule such as DNA or protein (DOH, 1991)
Agent is any chemical, physical, biological or social substance or factor being assessed, unless otherwise noted
BMD (Benchmark Dose) is the dose associated with a given incidence (eg. 1%, 5% or 10% incidence) of effect, the Benchmark Risk, based on the best-fitting dose-response curve.
BMR (Benchmark Risk) is a predetermined incidence of adverse response which determines the Benchmark dose.
Background Concentration is the naturally occurring, ambient concentrations of substances in the local area of a site.
Bioavailability is a unitless measure of the ratio of the amount of chemical exposure (applied dose) and the amount of chemical that enters the tissues of exposed biota (absorbed dose).
Biological Monitoring is the measurement of a contaminant or metabolite in body tissue or fluid. It is usually used as a marker or indicator of exposure to environmental chemicals.
Biota are plants, animals, including humans, fungi or bacteria.
Cancer is a disease of heritable, somatic mutations affecting cell growth and differentiation. That is, genetic alterations incurred in the first damaged cells are acquired in subsequent cells after cell division within the same individual.
Carcinogen is a cancer-causing agent.
Carcinogenesis is the origin, causation and development of tumours. The term applies to all forms of tumours (eg. benign and malignant).
Confidence is the weight assigned by the evaluator to the quality of the information available (high, medium or low confidence) to indicate that a chemical possesses certain toxicological properties.
Confidence Limits are the range of values determined by the degree of presumed random variability in a set of data, within which the value of a parameter, eg. the mean, lies, with a specified level of confidence or probability (eg. 95%). The confidence limit refers to the upper or lower value of the range (DOH, 1991).
Confounding Factor is a factor that distorts the apparent effect or magnitude of the effect of a study factor or risk. Such factors must be controlled for in order to obtain an undistorted estimate of a given effect (DOH, 1991).
Contamination is the condition of land or water where any chemical substance or waste has been added at above background and represents, or potentially represents, an adverse health or environmental impact.
Critical Effect(s) is the adverse effect(s) judged to be most appropriate for determining the tolerable intake.
Default Value is a pragmatic, fixed or standard value used in the absence of relevant data.
Dermal is of the skin, through or by the skin.
DNA is the carrier of genetic information for all living organisms except for the group of RNA viruses.
Dose is the total amount of a chemical that enters or interacts with a receptor (biota including humans). The applied dose is the amount of chemical in contact with the primary absorption boundaries (eg. skin, lungs, gastrointestinal tract) and available for absorption. The absorbed dose is the amount crossing a specific absorption barrier (eg. the exchange boundaries of skin, lung, and digestive tract) through uptake processes. The amount of the chemical available for interaction by any particular organ or cell is termed the delivered dose of that organ or cell (US EPA 1992, p 22933).
Dose-response is the relationship between the dose of a chemical and the extent of the toxic effect produced by the chemical in a biological system.
Endpoint is an observable or measurable biological event used as an indicator of the effect of a chemical on a biological system (cell, organism, organ etc.).
Epidemiology is the study of the distribution and determinants of disease frequency in human populations.
Exposure is contact of a chemical, physical or biological agent with the outer boundary of an organism, such as by inhalation, ingestion or dermal contact.
Exposure Assessment is the estimation (qualitative or quantitative) of the magnitude, frequency, duration, route and extent (for example, number of organisms) of exposure to one or more contaminated media.
Exposure Pathway is the course a chemical or physical agent takes from a source to an exposed organism. An exposure pathway describes a unique mechanism by which an individual or population is exposed to chemicals or physical agents at or originating from a site. Each exposure pathway includes a source or release from a source, an exposure point, and an exposure route. If the exposure point differs from the source, a transport/exposure medium (eg. air) or media (in cases of intermedia transfer) also is indicated. (US EPA, 1989, p. 62)
Exposure Route is the way a chemical enters an organism after contact eg. by ingestion, inhalation, or dermal absorption (US EPA 1992).
Extrapolation means for dose-response curves, an estimate of the response at a point outside the range of the experimental data. Also refers to the estimation of a response in different species or by different routes than that used in the experimental study of interest.
Factor means a single factor or product of several single factors by which the modified-benchmark dose is divided to derive an acceptable intake. These factors account for adequacy of the study, interspecies extrapolation, inter-individual variability in humans, adequacy of the overall data base, nature and extent of toxicity, public health regulatory concern and scientific uncertainty.
Gene means the functional unit of inheritance.
Genotoxic means the chemical agents for which the primary biological activity is the alteration of the information encoded in genetic material (Butterworth, 1990).
Guidance Values are the values such as concentrations in air or water, which are derived after appropriate allocation of Tolerable Intake (TI) among the possible different media of exposure. Combined exposure from all media at the guidance values over a lifetime would be expected to be without appreciable health risk. The aim of a guidance value is to provide quantitative information from risk assessment for risk managers to enable them to make decisions concerning the protection of human health." (WHO, 1994, p16)
Guideline Dose means the average daily intake of a chemical which, for a life-time, should not result in cancer, based on a comprehensive expert assessment of the best information available at the time. The guideline dose is derived by regulatory authorities using cancer risk assessment according to guidelines developed by national health advisory bodies.
Hazard is the capacity of an agent to produce a particular type of adverse health or environmental effect eg. One hazard associated with benzene is that it can cause leukemia; one hazard associated with DDT is that it can cause the thinning of eggshells of some predatory birds.
Health Investigation Level is the concentration of a contaminant above which further appropriate investigation and evaluation will be required.
Health Risk Assessment is the process of estimating the potential impact of a chemical, biological, physical or social agent on a specified human population system under a specific set of conditions and over a certain timeframe.
Health Risk Management is the process of evaluating alternative actions, selecting options and implementing them in response to health risk assessments. The decision making will incorporate scientific, technological, social, economic and political information. The process requires value judgements eg. on the tolerability and reasonableness of costs.
IRIS (Integrated Risk Information System) is the computerised database of the US EPA which provides the Agency’s adopted hazard and dose-response assessment for chemical and radiological agents. Used as guidance and to provide consistency in the Agency’s regulatory decisions designed to reduce risk related to environmental exposures (see abbreviations).
Life-time covers the average life span of an organism (eg. 70 years for humans).
LED10 (Lowest Effective Dose) means the lower 95% confidence limit on a dose associated with an estimated 10% increased tumour or relevant non-tumour response (US EPA, 1996).
LOAEL (Lowest Observed Adverse Effect Level) is the lowest concentration or amount of a substance, found by experiment or observation, that causes adverse alterations of morphology, functional capacity, growth, development or life span of target organisms.
Metabolite is a substance which is the product of biochemical alteration of the parent compound in an organism.
Metastasis is the transfer of abnormal cells or pathogenic microorganisms from one organ to another in the body.
Model is a mathematical representation of a biological system intended to mimic the behaviour of the real system, allowing description about empirical data and predictions about untested states of the system.
NOAEL (No Observed Adverse Effect Level) is the greatest concentration or amount of a substance, found by experiment or observation, which causes no detectable adverse alteration of morphology, functional capacity, growth, development or life span of the target organism under defined conditions of exposure. Alterations of morphology, functional capacity, growth, development or life span of the target organism may be detected which are judged not to be adverse.
Public Health is the science and art of preventing disease, prolonging life and promoting health through organised efforts of society.
PTWI (Provisional Tolerable Weekly Intake) is the intake of a chemical deemed to be tolerable expressed as a weekly amount. The term was established by WHO (1972) for several heavy metals which 'are able to accumulate within the body at a rate and to an extent determined by the level of intake and by the chemical form of the heavy metal present in food.' (WHO, 1989)
QRA (Quantitative Risk Assessment) is a risk assessment procedure which yields numerical descriptors of risk.
Response Level is the concentration of a contaminant at a specific site, based on a site assessment, for which some form of response is required to provide an adequate margin of safety to protect public health and/or the environment.
RfD (Reference Dose) is an estimate (with uncertainty factors spanning perhaps an order of magnitude) of the daily exposure (mg/kg/day) to the general human population (including sensitive sub-groups) that is likely to be without an appreciable risk of deleterious effects during a life time of exposure. It is derived from the NOAEL or the LOAEL by application of uncertainty factors that reflect various types of data used to estimate RfD and an additional modifying factor, which is based on professional judgement of the entire data base of the chemical (IRIS, 1996).
Risk is the probability in a certain timeframe that an adverse outcome will occur in a person, a group, or an ecological system that is exposed to a particular dose or concentration of a hazardous agent, ie. it depends on both the level of toxicity of the hazardous agent and the level of exposure.
Safety factors are the numerical values used to divide the LOAEL or NOAEL when deriving acceptable intakes and account for the adequacy of the study, interspecies extrapolation, inter-individual variability in humans, adequacy of the overall data base, nature and extent of toxicity, public health regulatory concern and scientific uncertainty. Safety factors usually refer to health-related concerns.
Site means the parcel of land being assessed for contamination.
Structure Activity Relationship is the relationship between the biological activity of chemicals or series of chemicals and their structure. The relationships can be described qualitatively and quantitatively.
Threshold Dose is the lowest dose which produces an effect and below which no biological effect is known to occur.
TDI (Tolerable Daily Intake) is an estimate of the intake of a substance which can occur over a lifetime without appreciable health risk. It is the TI expressed as a daily amount. (Imray and Langley, 1996, p18) It may have different units depending on the route of administration (WHO, 1994).
TI (Tolerable Intake) is an estimate of the intake of a substance that over a lifetime is without appreciable health risk. (WHO, 1994)
Toxicity is the quality or degree of being poisonous or harmful to plant, animal or human life.
Transformation is the process by which a normal cell acquires the capacity for neoplastic or carcinogenic growth. It is thought to occur in several stages.
Tumour is a mass of abnormal, disorganised cells, arising from pre-existing tissue, which is characterised by excessive and uncoordinated cell proliferation or growth and by abnormal differentiation (specialisation). There are two types of tumours, benign and malignant. Benign tumours morphologically resemble their tissue of origin, grow slowly (may also stop growing) and form encapsulated masses; they do not infiltrate other tissues, they do not metastasise and are rarely fatal. Malignant tumours resemble their parent tissue less closely and are composed of increasingly abnormal cells genetically, morphologically and functionally. Most grow rapidly, spread progressively through adjacent tissues and metastasise to distant tissues.
Tumour Initiation is the first step in carcinogenesis whereby a small number of cells (or one cell) are irreversibly changed due to genetic damage.
Tumour Progression is the stage in carcinogenesis when tumours acquire the features of malignant growth.
Tumour Promotion is the process by which initiated cells undergo clonal expansion to form overt tumours.
Uncertainty is the lack of knowledge about the correct value, eg a specific exposure measure or estimate.
Variability relates to measurable factors that differ eg height is variable across populations. The major types of variability are temporal, spatial and interindividual. They may be discrete (eg albinism) or continuous (eg body weight). It may be readily identifiable (eg presence of albinism) or difficult to identify (eg ability to detoxify a particular chemical metabolite).
4. FRAMEWORK FOR RISK ASSESSMENT
There are various models available for the health risk assessment of contaminated sites. The principal forms are those of the US EPA (1989) and the National Academy of Sciences (1983).
Risk assessment is intended "to provide complete information to risk managers, specifically policymakers and regulators, so that the best possible decisions are made" (Paustenbach, 1989, p28).
The process of risk assessment is intended to achieve the following objectives when assessing contaminated sites (US EPA 1989):
• to establish baseline risks and whether site remediation or other action is necessary;
• to determine a tolerable level of contaminants that can remain in place with adequate protection of public health;
• to enable comparison of potential health impacts of various remediation techniques; and
• to provide a consistent method of appraising and recording public health risks at sites.
The framework of risk assessment involves four stages which are described by US EPA, (1989)1 as follows, and these stages are used to describe the process of health risk assessment in this document:
• data collection and evaluation of the chemical condition of the site;
• toxicity assessment of contaminants;
• exposure assessment for the population on or near the site;
• risk characterisation. (Some texts use 'risk assessment' only to refer to risk characterisation)
The relationships between these stages are detailed in Figure 4-I.
Data collection entails the acquisition and analysis of information about chemicals on a site that may affect human health and which will be the focus for the particular risk assessment.
Toxicity assessment considers:
• the nature of adverse effects related to the exposure;
• the dose-response relationship for various effects; and
• the weight of evidence for effects such as carcinogenicity.
![]() 1 The National Academy of Sciences model (1993) has four stages generally similar to those of the US EPA: hazard identification, dose-response, exposure assessment and risk assessment.
1 The National Academy of Sciences model (1993) has four stages generally similar to those of the US EPA: hazard identification, dose-response, exposure assessment and risk assessment.
Both qualitative and quantitative toxicity information is evaluated to estimate the incidence of adverse effects occurring in humans at different exposure levels.
Exposure assessment involves estimating the frequency, extent and duration of exposures in the past, present, and in the future. It also identifies exposed populations and particularly sensitive sub-populations, and potential exposure pathways. Environmental monitoring and predictive fate and transport models can be used to determine the levels of exposure at particular points on the exposure pathways. The contaminant intakes from the various pathways under a range of scenarios can then be estimated.
Given this information, risk characterisation details the nature and potential incidence of effects for the exposure conditions described in the exposure assessment. An integral part of this stage is to evaluate the uncertainties and assumptions in the risk assessment process.
Figure 4-I
Risk assessment model

adapted from US EPA, 1989.
The role of site-specific health risk assessment in the process of the assessment of contaminated sites is outlined in Figure 4-II.
In conducting risk assessments two guiding principles are recommended (US EPA 1995, p2):
1. Risk assessors and risk managers should be sensitive to distinctions between risk assessment and risk management.
The assessors should:
• generate a credible, objective, realistic, and scientifically balanced analysis;
• present information on the separate components of the risk assessment; and
• explain the confidence in each assessment by clearly delineating strengths, uncertainties and assumptions, along with the impacts of these factors (eg confidence limits, use of conservative/non-conservative assumptions) on the overall assessment.
The risk assessors should do this without considering issues such as cost, feasibility, or how the scientific analysis might influence the regulatory or site-specific decision.
Figure 4-II
Site-specific Health Risk Assessment in the Assessment of Contaminated Sites
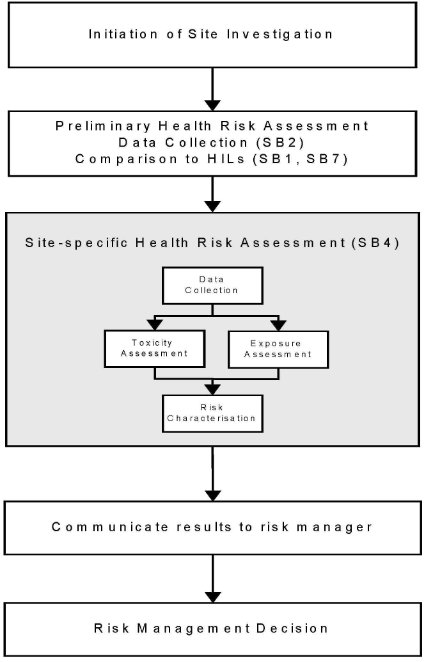
Note:
1. Occupational Health & Safety issues for workers involved in site assessment are the subject of Site-Specific Safety Plans (see Schedule B(9))
2. Community consultation may be needed before risk management decisions are taken in some situations (see Schedule B(8))
2. Risk characterisation is only one of several kinds of information used for decision-making. The risk management decision will be determined not only by the risk assessment but a range of other factors including "technical feasibility (eg treatability, detection limits), economic, social, political," and legislation when determining whether to regulate and, if so, to what extent.
It is important that the basis of the decision-making is clearly documented.
5. DATA COLLECTION
5.1 Introduction
Data collection entails the acquisition and analysis of information about chemicals on a site that may affect human health and which will be the focus for the particular risk assessment (US EPA, 1989). The purpose of data collection is to gather data that will enable a useful risk assessment to be undertaken eg data related to contaminant types and distributions and the potential environmental behaviour of the contaminants.
Data Collection is detailed in Schedule B(2).
Adequate data collection is the foundation to an acceptable health risk assessment.
The data collection phase will comprise the following components as described in Schedule B(2).
1. Setting Data Quality Objectives (see Section 2.3)
2. Establishing a Site History (see Section 2.4)
3. Detailing the Proposed Use (see Section 2.2)
4. Establishing a Sampling strategy and sampling pattern (see Sections 3.1 – 3.5)
5. Ensuring appropriate analysis (Choice of Analytes) (see Sections 3.6)
6. Coherent presentation of the data (Assessment of summary statistic data ) (see Sections 5.1 – 5.2)
Before any sampling is undertaken, the objectives of the task should be defined
The greatest concern, in collecting soil and water samples, is to ensure that the samples taken represent all the waters, and the soils in all strata present on the site. Consequently it is essential to be fully apprised of the conditions at the site locations and what analytes will be tested in each sample, before sampling commences. (Lock 1996, p2)
6. EXPOSURE ASSESSMENT
6.1 Introduction
Exposure assessment involves the estimation of the magnitude, frequency, extent and duration of exposures in the past, currently, and in the future. It also identifies exposed populations, and particularly sensitive sub-populations, and exposure pathways.
The process involves:
• Analysis of contaminant releases;
• Identification of exposed populations;
• Identification of potential exposure pathways;
• Estimation of exposure concentrations for each pathway; and
• Estimation of contaminant intakes for each pathway for a range of scenarios.
Direct measurement of the exposures of the (potentially) affected population provides the best exposure data but this is not always available or practicable and default exposure factor data is often required.
(Langley 1993, p90)
The following issues are relevant to exposure assessment
• The use of point estimates and probability distributions including Monte Carlo-type methods (Section 6.3 below);
• Information to assist in interpretation of results (Section 6.4 below);
• Exposure comparisons to values such as ADIs (Section 6.5 below);
• Biological monitoring - Direct measure of exposure (compared with exposure modelling and environmental modelling) (Sections 6.6 –6.8 below);
• Health monitoring (Section 6.9 below);
• Default exposure settings, encompassing a range of exposure settings (Sections 6.10 below– 6.11 below);
• Matters to be addressed in exposure assessment reports (Sections 6.12 below -6.13 below)
• Default values to be used in the absence of site specific data (Section 6.14 below);
• Further sources of exposure data (Section 6.15 below).
Accurate and useful exposure assessment requires a detailed understanding both of the strengths and weaknesses of the exposure assessment techniques, and the specific exposure factors used in the assessment.
6.2 Key Points in Exposure Assessments of Contaminated Sites
1. Children usually receive a higher exposure to soil contaminants per unit body weight than adults;
2. Soil ingestion by small children is usually by far the most important exposure route;
3. One exposure route will normally predominate;
4. The inhalation route will be important for highly volatile contaminants but, as they rapidly evaporate, they will rapidly disappear from a site unless new sources are added;
5. In large-scale contamination (ie regional) more exposure pathways will be involved than in small-scale (very localised) contamination.
6. All exposure pathways must be considered for health risk assessment. Existing Australian data for other exposure pathways eg. contaminant levels in food, water and air need to be appraised to enable comparisons.
7. Direct dermal and inhalation exposure pathways should form the basis of occupational health risk assessment. Existing Australian Time-weighted Average (TWA) and Biological Exposure Indices standards should be considered for this purpose.
(ECETOC, 1990; Langley, 1991)
6.3 Use of Point Estimates and Probability Distributions
6.3.1 Introduction
Point estimates are most commonly used in Australia for exposure assessments. A point estimate is a single value chosen to represent a population eg 70kg as the weight of an adult. Point estimates are usually typical values for a population or an estimate of an upper end of the population's value eg 70 years as the duration of residence on a property. An upper end value may be chosen for reasons of conservatism and/or to provide a "worse case" scenario.
Where a risk assessment uses a series of upper end estimates, the result can be a worse than worse case scenario due to the compounding effects of the estimates eg the person with the upper end value for weight is unlikely to have; the upper end value for water consumption that has the upper end value for contamination; the upper end value for duration of residence; the upper end value for soil ingestion, etc.
In recent years there has been increasing attention paid to the use of Monte Carlo-type exposure assessments and such methods have been acknowledged by the US EPA and the UK Department of the Environment (US EPA, 1992a; Ferguson et al, 1994).
These methods are 'more informative and inherently more representative' (Ruffle et al, 1994, p 403) than point estimates. If applied appropriately point estimates still have a major role in exposure assessment as they are readily understood and
applied, and may incorporate safety factors that could be lost with Monte Carlo-type exposure assessments.
The Monte Carlo-type exposure assessments rely on the use of probability distribution functions. A distribution of possible values for each of the parameters (is) described …. along with the probability of occurrence of each. Using standard mathematical formulae several thousand iterations of a mock mathematical model are performed. For each iteration, values for each parameter are selected randomly from each distribution based upon the probability of occurrence. The estimated risk values are combined to provide a frequency distribution of possible risk (Alsop et al., 1993). Figure 6-I demonstrates the process of the Monte Carlo method. The end result is a more representative picture of the range of exposures, and hence risks, in a population than occurs with the use of point estimates.
If a Monte Carlo assessment is performed the methodology must be 'transparent' or problems will arise in community consultation. As with any form of risk assessment, the basic principles of the method must be able to be understood by the affected community.
On a small site, the use of Monte Carlo methods is likely to be to complex and/or costly and it may be more appropriate to do direct measurements of exposure. The exposures of 'outliers' must always be acknowledged in risk assessments and ways of identifying and accommodating them must be considered.
This is particularly important in the assessment of an existing site contamination (eg. a site where housing has already been developed), rather than a forecast exposure scenario, where the presence of outliers will severely affect the credibility in any risk communication exercise. (Langley and Sabordo 1996)
Figure 6-I
Principles of the Monte Carlo Method

(adapated from Ferguson 1994))
6.3.2 Weaknesses With the Monte Carlo Technique
Langley and Sabordo (1996) reviewed the key limitations of the Monte Carlo technique. The limitations include:
6.3.2.1 Complexity
While the Monte Carlo method has a very general applicability changing one variable may mean large amounts of re-calculation because of the extent of the iterative process when using this model. The complexity reduces the 'transparency' of the method. This may create difficulties in community consultation and risk communication, it obscures errors, and creates difficulties for checking by both the modellers and administering authorities.
6.3.2.2 Loss of factor distinctions
The method does not indicate which variables are the most important contributors to output uncertainty. (US EPA, 1992).
6.3.2.3 Unrealistic probability assessments
US EPA (1992) notes that simulations such as that found with the Monte Carlo model often "include low probability estimates at the upper end that are higher than those actually experienced in a given population, due to improbability of finding these exposures or doses in a specific population of limited size, or due to nonobvious correlations among parameters at the high ends of their ranges". This results in overestimations of exposure dose or risk. The Science Advisory Board of the US EPA has noted that "for large populations, simulated exposures, doses and risks above the
99.9 percentile may not be meaningful when unbounded lognormal distributions are used as a default". (ibid, p22922)
6.3.2.4 Assessment endpoints
With Monte Carlo-type assessments there is still a need to determine what is an acceptable level of exposure. Smith (1994) considers that 'the level of exposure exceeded by 1 in 20 exposed persons would seem to be an appropriate reasonable maximum'. This would allow 5% of the population not to be included in the exposure assessment.
6.3.2.5 Variability-uncertainty confusion
Smith (1994) highlights the need to distinguish between 'variability' (measurable factors that differ across populations such as height) and 'uncertainty' (unknown, difficult to measure factors such as frequency of trespassing on a site). Currently available software packages do not distinguish between variability and uncertainty. An administrator reviewing a Monte Carlo risk assessment will, however, need to appreciate the differences between variability and uncertainty and the nature and extent of both.
6.3.2.6 Limited exposure data
Limited information is available about many variables for the exposure assessments. As a consequence of this, many input variables are described as triangular distributions. Smith (1994) stresses the need 'to collect and verify distributions from many currently undescribed input assumptions' to improve accuracy.
The use of Monte Carlo methods may be inappropriate where the predictions of exposure are so dominated by uncertainties. McKone (1994) gives the example of benzo(a)pyrene, where information on benzo(a)pyrene exposure is 'not readily available' so that the use of Monte Carlo methods to assess variability in population exposures is somewhat redundant.
6.3.2.7 Simplification of complex situations
Exposure assessments are comprised of combinations of modelling, sampling, and modelling/sampling combinations. Even the use of complex models still provides a static picture of a dynamic world albeit a more elaborate representation of reality (McKone, 1994) and such a picture must be placed within a sound theoretical framework.
6.3.2.8 Misleading precision
The use of more complex models 'does not necessarily increase precision'. The costs of collecting and analysing data, and constructing new models 'must be balanced by the value of the information obtained' . There is a need to appraise the value of information along with its uncertainties in 'defining the capabilities and limits of exposure models'. (McKone, 1994)
6.3.3 Characterisation of Extreme Values
The 50th percentile can always be estimated with less uncertainty than the 99th percentile (Finley et al, 1994). Problems in estimating the extreme percentiles can come from limitations in the measurement techniques (eg incorrect and implausible estimates of dietary consumption may be accepted into the survey); the duration over which exposure data was collected (see short term and long term variation, above); and whether there are sub-populations who may have unusual exposures (eg. vegetarians, subsistence fishermen) (Finley et al, 1994)
6.3.4 Selecting Appropriate Data Sets
For describing a probability distribution, the relevant studies and the quality of the data produced may vary considerably. Unless data sets are rigorously scrutinised 'the resulting uncertainty in the range of risk estimates could be greater than obtained using point estimates' (Finley et al, 1994, p 536)
6.3.5 Principles For The Use Of Monte Carlo-Type Techniques
Burmaster and Anderson (1994) stress that any method of exposure assessment must have a 'clearly defined assessment end point' and provide all relevant information so that the assessment can be reproduced and evaluated. They detail fourteen principles for good practice in Monte Carlo assessments. These are:
1. Detail all formulae.
2. Detail point estimates of exposure where these are demanded by regulatory agencies.
3. Detail sensitivity analyses to enable the identification of relevant and important input variables. Those variables which will drive the risk assessment must obviously be included in the Monte Carlo analysis but reasons for excluding insignificant variables must also be detailed.
4. Use probabilistic techniques (which may be demanding in terms of time, money and other resources) only where exposure pathways are likely to be significant.
5. Provide detailed information about input distributions with the minimum being:
• a graph showing the full distribution and the location of the point value used in the (point estimate) risk assessment;
• a table showing the mean, standard deviation, the minimum (if one exists), the 5th percentile, the median, the 95th percentile, and the maximum (if one exits)' (p 478). There needs to be a sufficient justification of the selected distribution which should be based on adequately referenced sources and the statistical, physical, chemical, and biological mechanisms relevant to the distribution.
6. Detail how the input distributions capture and represent both the variability and the uncertainty in the input variables' (p 478) so as to enable both variability and uncertainty to be described and analysed separately.
7. Use measured data to test the relevance of the input distribution to the population, place and time of the exposure assessment. Further data may need to be gathered to supply missing information or supplement incomplete information.
8. Describe the methods by which measured data were used to derive a probability distribution.
9. Detail any correlations between data where there are relatively high correlations. Sensitivity analysis may be necessary to determine the effects of correlations between variables on the exposure analysis.
10. Provide detailed information and graphs for each output distribution. Burmaster and Anderson suggest the following as a minimum:
• a graph of the variable with administratively set allowable risk criteria (if these are available) as annotations and point estimates of risk using the administratively set point estimates of exposure;
• A table of the mean, the standard deviation, the minimum (if one exists), the 5th percentile, the median, the 95th percentile, and the maximum (if one exists)' (p 479).
11. Provide records of sensitivity analyses which will enable the determination of most important input variables (or groups of variables).
12. Assess the numerical stability of the central moments (mean, standard deviation, skewness, and kurtosis) and the tails of the output distributions. The latter are particularly sensitive to the nature of the tails of the input distributions and, as they stabilise very slowly, sufficient iterations are required to demonstrate the numerical stability. Burmaster and Anderson suggest that commonly more than 10 000 iterations are required. Software that enables Latin hypercube sampling results in more rapid stability of these output tails. Burmaster and Anderson state that 'the changes in the tails of only a few input distributions contribute strongly to changes in the upper tail of the output distribution' (p 480).
13. Detail the name and statistical quality of the random number generator used. Some generators are inadequate because of short recurrence periods.
14. Interpret the results and detail the limitations of the methodology such as the effects of biases not elsewhere interpreted.
Burmaster and Anderson state that the principles are not mutually exclusive nor collectively exhaustive."
6.3.6 Administrative Requirements for the Use of Monte Carlo Methods
Regulatory authorities in Australia will require assessments using Monte Carlo methods to meet the following criteria:
• Meeting the 14 principles of Good Practice detailed above.
• The provision of adequate information to the authority to enable review of the assessment. This may require the provision of the software (and underlying formulae) and data.
• A demonstration of the relevance of the exposure data to the site: data from other countries or cultural backgrounds may not be relevant.
• An explanation of the data and method which will be able to be understood by the relevant community.
• The use of data that accounts for age and gender differences and takes into account susceptible populations.
On a large site divided into housing lots, the results for specific housing lots that may be affected by atypically elevated concentrations should not be obscured by averaging or Monte Carlo techniques applied to the entire site. In many instances, Monte Carlo methods will only be relevant to large sites or sites where direct measurements of exposure are not practicable.
Government authorities will need to define the range of acceptable exposures. Given that the Monte Carlo method loses much of the conservatism usually inherent in point estimates and hence the safety factors, it is proposed that the 99th percentile of exposures for particular groups (eg. by age: young children; children; and adults, and by susceptibility eg. asthmatics) be chosen. Depending on the conservatism of the toxicological assessment, this should result in adequate protection for at least 99% of the population. It should be noted that for a population of 1 million, 10 000 people would exceed the 99th percentile for exposure." (Langley and Sabordo 1996, p141)
6.4 Appraising Exposure Assessments
Factors that tend to result in underestimates of exposure (EPA, 1992):
• Overlooking a significant exposure or metabolic pathway.
• Failure to evaluate all contaminants of concern in the mixture.
• Comparison of exposure-related data against contaminated media or exposed populations rather than against appropriate background levels.
• Using insufficiently sensitive detection limits so that meaningful values are reported as not detected.
• Relevant individual pathways within the same exposure route may not have been summed.
Factors which can cause overestimates of exposure include (EPA, 1992):
• The use of unrealistically conservative exposure parameters.
• Portraying hypothetical potential exposures as existing exposures.
• Attributing a significant value to results that fall below an appropriate detection limit. Substituting such values may create the impression of values where none exist.
Factors that may cause underestimates or overestimates include (EPA, 1992):
• Computational errors.
• Use of inappropriate factors eg. for intake routes.
• Insufficient uncertainty assessment to put the exposure assessment in perspective.
• Use of an inappropriate number of significant figures for the numeric estimates in a situation where using more than one significant figure may imply more confidence in the results than is warranted.
• The unthinking and uncritical use of models. While the concept of "garbage in, garbage out" is well accepted, some risk assessment models result in "quality in, garbage out" (see Calabrese and Kostecki, 1992).
• The failure to take into account correlations among input distributions when using simulations such as Monte Carlo. It will be unnecessary to use Monte Carlo simulation if the relationship between variables is known.
6.5 Exposure Durations and Exceedances of Acceptable Daily Intakes (ADIs)
Appropriate durations of exposure need to be assessed so that transient (short term) and important exposures are not obscured by the use, for example, of average lifetime exposures. This is important in the Australian context where Acceptable Daily Intake values from WHO have been used to establish Health-based Investigation Levels. The duration and magnitude of exceedances of the ADIs must be obvious in exposure assessments.
6.6 Biological Monitoring
Biological monitoring (based on Langley, 1991a) is a measuring procedure whereby validated indicators of the uptake of contaminants, or their metabolites, and people's individual responses are determined and interpreted. Whereas environmental monitoring measures the composition of the external environment around a person, biological monitoring measures the amount of contaminant absorbed into the body.
If biological monitoring is practicable it will be more valuable than environmental monitoring in determining the level of risk from an environment as it will measure whether exposure is occurring and the level of exposure.
The biological samples used for monitoring include: blood, urine, fat, hair, and expired air.
Biological monitoring should not be commenced before:
• The objective of the biological monitoring is defined clearly.
• A normal range of results is established that is applicable for the population under study.
• Consideration has been given as to how people with abnormal results are to be managed.
• A centralised collection point for results has been established to enable consistent analysis and epidemiological appraisal of results.
Results should always be available to participants in biological monitoring with an explanation of the results.
Several aspects must be considered:
• A good biological monitoring test may not correlate well with environmental levels (mainly because of human behavioural and toxicokinetic factors).
• The number of substances which can be used reliably for biological monitoring is still small.
• Irritative, locally or rapidly acting substances are usually unsuitable as the systemic absorption may be minimal and/or irrelevant to the level of local reaction (eg. SO2, ammonia, direct skin exposure to PAHs causing skin cancer).
• The substance must be in some tissue or fluid suitable for sampling.
• Accurate, valid and practical measuring methods must be available.
• The result should be interpretable in terms of health risk.
• The results may have more value for a group than an individual.
6.7 Choice of a Test
Optimally, a biological monitoring test (based on Langley, 1991) would give a result which reflected the exposure, the concentration of the substance in the target organ and the risks of adverse effects (Friberg, 1985). Few tests are available which approach this ideal (Langley et al 1998).
In Australia, exposures from contaminated soil will be generally low, creating problems in accurate measurement at low levels and the possibility of results being overwhelmingly influenced by other sources of exposure (eg. the influence of cadmium in food, tobacco smoke and the occupational environment will generally be far greater than the influence of cadmium contamination of soils).
For many substances, biological monitoring is impracticable because:
• Analytical techniques are not available or are inaccurate at low levels or in the tissues or fluids being tested.
• Insufficient information is available on inter- and intra-individual toxicokinetics and thresholds of health effects to enable risk assessment of results.
• Insufficient epidemiological studies have been done to determine normal ranges.
Hair is an inappropriate tissue for biological monitoring on or near contaminated sites. External contamination of the hair cannot be adequately removed during sample preparation and an accurate measure of excretion via hair cannot be performed. Hair analysis may be useful for assessing intake from purely dietary sources when there is no general environmental contamination.
Substances for which biological monitoring of general environmental exposures is practicable are detailed in Table 6-A.
Table 6-A
Substances likely to be suitable for biological monitoring
Substance | Fluid/ Tissue | Comments |
Lead | Blood | Urinary lead does not accurately reflect either recent exposures or body burden. Substantial data available on level of risk for particular blood lead ranges. Numerous Australian studies which provide comparison data. Levels of concern available for both general population and occupational groups (WHO, 1986, NHMRC, 1987). |
Cadmium | Urine/ Blood | Urinary levels tend to reflect body burden, blood levels reflect recent exposures. Urinary levels need to be adjusted for changes in urinary flow rates (results often given as ugCd/g Creatinine or ugCd/24 hr). Laboratory inaccuracy has always been a major problem, particularly prior to 1980. Limited Australian studies to provide comparison data. Most international studies have concentrated on occupational exposures. Very limited data on children, especially for those less than 5 years. World Health Organisation (cited in Mueller et al, 1989) has set levels of concern. General diet and smoking will tend to have a major influence on levels. |
Arsenic | Urine | Short biological half life - study must be done during exposure (or at most within 1 – 2 days afterwards). Considerable interference from organic sources of arsenic (eg. seafood) - dietary sources from environment not under study need to be excluded and testing for inorganic arsenic undertaken. Limited comparison data and no set levels of concern. |
Mercury | Blood, Urine | At equilibrium, the concentration of mercury in the blood reflects daily intake and is probably the single best indicator of exposure. This measure will also include methylmercury from fish and a fractionated analysis of mercury salts and alkylmercuric compounds may be required (Aitio et al, 1988). Methylmercury exposure will not affect urinary mercury levels although urinary levels show significant diurnal variation. Some international comparison data is available (ibid). |
Substance | Fluid/ Tissue | Comments |
Polychlorinated biphenyls (PCBs) | Blood, Adipose tissue (fat) | Long biological half-life so that historical exposures (ie. body burden) may be able to be monitored. Different PCBs will have different behaviours in the body and different biological half-lives. Some comparison data available. It is difficult to obtain adipose tissue samples and blood sampling is usually preferred. |
Organochlorine pesticides eg. aldrin, dieldrin, chlordane, heptachlor | Blood, Adipose (fatty) tissue | Long biological half-life so that body burden can be assessed. Some comparison data available, especially for blood. It is difficult to obtain adipose tissue samples and blood sampling is usually preferred. |
Organophosphorus pesticides | Blood | Cholinesterase levels will enable physiological response to be monitored. Wide range of normal values require individual baseline values to enable an assessment of "normality". |
Adapted from Langley (1991a)
Most organic contaminants are not amenable to biological monitoring in general environmental situations because of the low levels of exposure and the lack of comparison data compared to occupational situations. Specialised studies may make biological monitoring of some inorganic substances practicable (eg. manganese, radioactive isotopes).
A good knowledge of the toxicokinetics of a substance is required for the correct choice of method and interpretation of results eg. individual results may be distorted if there is not constant exposure or equilibrium within the body.
Under the NOHSC National Model Regulations for the Control of Workplace Hazardous Substances (adopted by the States and Territories), health surveillance is required for specified substances. Biological monitoring methods developed for some of these methods are detailed in the NOHSC Guidelines for Health Surveillance.
6.8 Biomarkers
The term 'biomarker' has been introduced recently and refers broadly to "almost any measurement reflecting an interaction between a biological system and an environmental agent, which may be chemical, physical or biological" (WHO, 1993). Three classes of biomarker are identified by WHO (1993, p12):
• biomarker of exposure: an exogenous substance or its metabolite or the product of an interaction between a xenobiotic agent and some target molecule or cell that is measured in a compartment within an organism;
• biomarker of effect: a measurable biochemical, physiological, behavioural or other alteration within an organism that, depending upon the magnitude, can be recognised as associated with an established or possible health impairment or disease;
• biomarker of susceptibility: an indicator of an inherent or acquired ability of an organism to respond to the challenge of exposure to a specific xenobiotic substance"
Some examples of commonly used biomarkers are serum cholinesterase for organophosphate exposure and serum enzymes for liver damage.
6.9 Health Monitoring
From Australian and international experience, health effects are likely to be found in only a very limited number of situations of extreme soil contamination. Subtle effects may only be able to be determined on a group basis rather than on an individual basis (eg. subtle neurodevelopmental effects determined by sophisticated testing in groups of children with different lead exposures). Similar problems of causation relating to individual findings rather than group findings arise if the putative effects are common in the general population eg. headache, fatigue. Health effects are rarely as specific to an exposure as chloracne with PCB or dioxin exposure, mesothelioma and asbestos exposure, and vinyl chloride monomer and haemangiosarcoma.
Health monitoring for specific health effects is warranted where environmental or biological monitoring has indicated a significant risk of effects eg. specific tests of renal function if urinary cadmium levels above the levels of concern are detected in biological monitoring.
When health monitoring is done it should rarely be done in isolation from environmental and/or biological monitoring. Clearly defined health effects should be sought with specific case-definition criteria. Records of other symptoms and clinical findings should also be kept to enable epidemiological assessment of other potential health effects. (Langley 1991a, p195)
6.10 Default Exposure Settings
Taylor and Langley (1998) Exposure scenarios and exposure setting details the derivation of default exposure settings and the qualifications for their use and these are provided in Schedule B(7B). It includes 'default exposure ratios' which are standard multiplication factors which can be applied to investigation levels for each setting to take into account expected differences in levels of exposure.
Table 6-B
Exposure Settings and Default Exposure Ratios for establishment of soil investigation criteria
(from Taylor and Langley 1998, p14)
Exposure Setting | Duration of exposure and age of exposed person | Default Exposure Ratio |
A. 'Standard' residential with garden and accessible soil. Home-grown food production contributing less than 10% of vegetable and fruit intake: includes daycare centres, kindergartens, preschools and primary schools | 70 years, commencing from birth | 1.0 |
B. Residential with vegetable garden (contributing ≥10% of vegetable and fruit intake ) and/or poultry | 70 years, commencing from birth | Not applicable: site and contaminant specific |
C. Residential with vegetable garden (contributing ≥10% of vegetable and fruit intake) Poultry excluded | 70 years, commencing from birth | Not applicable: site and contaminant specific |
D. Residential with minimal opportunity for soil access | 70 years, commencing from birth | 0.25 |
E. Parks, recreational open space, playing fields: includes secondary schools | 70 years, commencing from birth | 0.5 |
F. Commercial/Industrial | 30 years, adults | 0.2 |
Notes to Table 6-A:
1. The default exposure ratios listed here are based upon judgement and designed to be conservative and protective of human health. They do not necessarily take into account environmental and aesthetic concerns, which may impact greatly upon remediation and management decisions. Therefore whilst an investigation level for commercial land use may be contemplated that is five times higher than that for residential land with garden, this may not be an acceptable investigation threshold from the perspective of protecting particular species or the ecosystem.
2. Health-based Investigation Levels have not been derived for exposure settings B and C because site-specific considerations need to be taken into account. In developing HILs for such sites, or conducting preliminary broad-based population risk assessments, it may be useful to refer to exposure assumptions detailed in Tables 8 and 9 in Taylor and Langley (1998).
3. For residential settings, it is assumed that 70 years is the duration of exposure. However for many contaminants (particularly those for which ADIs or PTWIs have been established) exposures over a much shorter period during childhood tend to dictate investigation criteria.
4. In the case of occupational exposure from a contaminated site currently used as a commercial or industrial site, it is assumed that 30 years is the duration of exposure.
5. These default exposure ratios should be seen as purely guideline values for development of soil investigation criteria rather than for derivation of soil response criteria.
6. Highly volatile substances are excluded from consideration in this table unless volatility has been taken into account in setting the HIL (see Taylor and Langley 1999, p 19).
7. National Occupational Exposure Standards have been developed with an undefined career duration.
"…Day-care centres and preschools (and primary schools to a lesser extent) potentially provide situations which are comparable to residential dwellings in terms of soil access by young children, and can be placed in the 'base case' residential setting.
Whilst inclusion of primary school sites in a 'residential' category may be seen as overly conservative in view of diminished mouthing behaviour and soil ingestion expected in this age group compared with infants and toddlers, primary school sites have been included in this category because some contain preschool or child-care centres; some contain special education units where children may be at increased risk of hand-mouth or pica behaviours; and social and community considerations about 'acceptable risk' have been taken into account in the regulatory framework. It is acknowledged that exposures in primary schools may be similar to exposures in secondary schools. If well-maintained barriers to soil access exist (eg in the form of paving such as cobblestones, gravel, or a substantial pine bark ground covering) then a primary school setting may not be comparable to a standard low-density 'residential with backyard garden' setting but more akin to high-density residential land use with reduced opportunities for soil access.
Similarly, a residence where the yard space is fully and permanently paved (eg concrete), or the contaminated soil is fully and permanently contained, affords minimal opportunities for contaminated soil access and investigation levels may be more appropriately considered in the context of a separate, lower-risk category.
A residential setting with accessible soil but minimal or negligible home food production has usually formed the baseline case for development of investigation levels to date in this country, but this approach has not explicitly quantified the home food production pathway.
It is advisable to distinguish those households with free-range poultry as special cases since this pathway may significantly influence exposure levels (Cross and Taylor 1994,1996). The great majority of urban local governments in recent times either prohibit poultry-keeping altogether, or require poultry to be kept on concrete pads where they remain out of contact with soil. If free-range poultry are being kept on contaminated soil then site-specific sampling of produce is likely to be the best means of determining exposure and the level of risk." (Taylor and Langley, 1998, p11)
6.11 Variation from Default Exposure Settings
Taylor and Langley (1998, p16) state: " For default assumptions not to be used, realistic and appropriately inclusive exposure opportunities for the proposed land use would need to be detailed, with sufficient safeguards for other potential future exposures. This may require annotations on the title documents or elsewhere stating the constraints on other possible land uses. The alternative exposure scenarios would have to differ markedly (possibly by an order of magnitude) from the defaults proposed here, in order for them to be used in preference to the defaults in establishing site-specific soil criteria."
A degree of conservatism is built into the default exposure settings as these relate to generic Health-based Investigation Levels that must provide for a wide range of scenarios within each default setting. To deal with uncertainties, some conservatism should remain when setting site-specific Response Levels, although it is anticipated that site-specific Response Levels will more closely reflect the site-specific exposure assessment.
6.12 Exposure Assessment of Volatile Contaminants
Volatile contaminants require specialised sampling techniques to ensure that the contaminants are not lost during and after sampling so that analytical results accurately represent the concentrations present on a site. The inhalation route will be more important than for non-volatile contaminants. It is often impractical to undertake environmental (ie air) sampling because of the constant variations over time of the concentrations as a result of fluctuation in temperature, wind speed and direction. Other factors that will have a significant effect are: soil disturbance; the physico-chemical properties of the soil and contaminants; and whether there is a renewable source or whether the contamination will dissipate over time. Exposure assessment will often depend on modelling. Models relevant to Australia are being developed by CSIRO in conjunction with the Environment Protection Authority (NSW) and the Public & Environmental Health Service in Adelaide. Preliminary details are provided in: Anderssen and Markey (1996); Anderssen and Markey (1997); Anderssen, de Hoog and Markey (1997); and, Anderssen and Markey (1998). Another methodology, “Guidelines for the Management of Hydrocarbon Impacted Land” is being developed by the Australian Institute of Petroleum. A process for the appraisal of the methodologies and determination of soil criteria may be considered as part of a future work plan that may arise from the Measure.
6.13 Exposure Assessment Reports
The following checklist details matters that should be appropriately addressed in an exposure assessment. Some material may be omitted, if justification can be provided. It is adapted from US EPA (1995):
1. What are the most significant sources of environmental exposure?
• Are there data on sources of exposure from different media? What is the relative contribution of different sources of exposure?
• What are the most-significant environmental pathways for exposure?
2. Describe the populations that were assessed, including the general population, highly exposed groups, and highly susceptible groups.
3. Describe the basis for the exposure assessment, including any monitoring, modelling, or other analyses of exposure distributions such as Monte-Carlo or krieging.
4. What are the key descriptors of exposure?
• Describe the (range of) exposures to: "average" individuals, "high end" individuals, general population, high exposure group(s), children, susceptible populations.
• How was the central tendency estimate developed? What factors and/or methods were used in developing this estimate?
• How was the high-end estimate developed?
• Is there information on highly-exposed subgroups? Who are they? What are their levels of exposure? How are they accounted for in the assessment?
5. Is there reason to be concerned about cumulative or multiple exposures because of ethnic, racial, or socioeconomic reasons?
6. Summarise exposure conclusions and discuss the following-,
• results of different approaches, ie. modelling, monitoring, probability distributions;
• limitations of each, and the range-of most reasonable values; and
• confidence in the results obtained, and the limitations to the results."
6.14 Default Values for Exposure Assessments
The following default values have been used in exposure models since 1991 to derive Health-based Soil Investigation Levels. They are adapted from Langley and Sabordo (1996, p184). These values should be used unless values more pertinent to the relevant population can be provided and justified. Factors should be relevant to the population about whom the exposure assessment is being done.
6.14.1 Dermal absorption factors
• Where available, substance specific data for bioavailability and dermal adherence should be used.
• A child's soil contact area will be equivalent to the area of both hands, both legs and both feet and this area of skin will be taken as 0.21 m2 (Hawley, 1985).
• The child will wash once each day.
• The soil adherence factor will be 11 mg per 21.5 cm2 (ibid) ie a total of 1 074 milligrams of soil on the exposed skin.
• Australian washing/bathing values are to be used where available.
6.14.2 Inhalation factors
• Inspirable particulates inside a house will be 75% of the level of inspirable particulates outdoors (Hawley 1985). US EPA (1989) found indoor airborne lead levels were 30% to 80% of outdoor levels for houses without air-conditioning.
• 75% of the inhaled dust will be retained in the respiratory tract and 25% will be exhaled (Hawley 1985).
• Half the inspirable dust will be sufficiently small to reach the pulmonary alveoli. This will be the respirable dust fraction and will be considered to have a diameter of less than 10 microns.
• Australian dust values are to be used where available.
Inhalation factors for particulates are dealt with in the NOHSC Exposure Standard Guidelines which are required by law for assessing occupational exposures.
6.14.3 Ingestion factors
• Where bioavailability data for ingested soil contaminants is unknown, the value of 100% absorption will be used. If bioavailability data are available it can be used providing the values are able to justified.
• Soil ingestion rates will be:
Age (years) | Soil Intake (mg/day) |
0-1 | Negligible |
1-5 | 100* |
5-15 | 50* |
Adult | 25* |
*conservative estimates from ANZECC/NHMRC(1992)
6.15 Sources of Exposure Assessment Data
Data must be pertinent to the relevant population. Where available, data from Australian populations are preferred.
Sources of information and data include:
Langley AJ. (1993). 'Refining exposure assessment.'
Langley AJ and Sabordo L (1996) Exposure Factors in Risk Assessment
Langley AJ, Taylor A and Dal Grande E (1998) 1996 Australian Exposure Factors
The Australian Bureau of Statistics can provide a range of Australian data.
The American Industrial Health Council's 'Exposure Factors Sourcebook' (1994) provides examples of probability distributions for a range of exposure factors. These largely relate to the US population. These, and similar US-based data, should only be used if they can be demonstrated to be relevant to the Australian population.
7. TOXICITY ASSESSMENT
7.1 Introduction
Toxicity assessment considers:
• the nature of adverse effects related to the exposure;
• the dose-response relationship for various effects;
• the weight of evidence for effects such as carcinogenicity; and
• the relevance of animal data to humans.
Both qualitative and quantitative toxicity information is evaluated in assessing the incidence of adverse effects occurring in humans at different exposure levels (US EPA, 1989).
There are two elements to the toxicological assessment: hazard identification and dose-response assessment.
Hazard identification examines the capacity of an agent to cause adverse health effects in humans and other animals. It is a essentially qualitative description based on the type and quality of the data, complementary information (eg structure-activity analysis, genetic toxicity, pharmacokinetic), and the weight of evidence from these various sources (US EPA, 1995). Key issues include (ibid):
• nature, reliability and consistency of human and animal studies
• the available information on the mechanisms of toxic effect; and
• the relevance of the animal studies to humans.
The dose-response assessment examines the quantitative relationships between exposure and the effects of concern. The determination of whether there is a hazard is often dependent on whether a dose-response relationship is present. Key issues include (US EPA, 1995):
• the relationship between the extrapolation models selected and available information on biological mechanisms
• how appropriate data sets were selected from those that show the range of possible potencies both in laboratory animals and humans
• the basis for selecting interspecies scaling factors to account for scaling doses from experimental animals to humans
• relevance of the exposure routes used in the studies to a particular assessment and the interrelationships of potential effects from different exposure routes
• the relevance to the assessment of the expected duration of exposure and the exposure durations in the studies forming the basis of the dose-response assessment
• the potential for differing susceptibilities in population subgroups.
The Toxicity Assessment phase is similar to the Hazard Identification and Dose Response stages in the National Academy of Sciences (1983) Risk Assessment model and other models.
The matters covered in the toxicity assessment phase of this document are:
• the components of a toxicological appraisal (Section 7.2 below)
• the sources and ranking of toxicological assessment data (Section 7.4 below)
• the methodology for establishing Guideline Doses as the basis of soil criteria for carcinogenic soil contaminants (Section 7.5 below).
7.2 Toxicological Appraisals
The following checklist is adapted with slight modification from US EPA (1995) and should be the basis of toxicological appraisals. A summarised version can be used if Tolerable Intake data from WHO or NHMRC are used as these matters should have been considered when the Tolerable Intake was set.
7.2.1 Hazard Identification
1. What is the key toxicological study (or studies) that provides the basis for health concerns?
• How good is the key study?
• Are the data from laboratory or field studies? In single species or multiple species?
• If the hazard is carcinogenic, comment on issues such as: observation of single or multiple tumour sites; occurrence of benign or malignant tumours; certain tumour types not linked to carcinogenicity; use of the maximum tolerated dose.
• If the hazard is other than carcinogenic, what endpoints were observed, and what is the basis for the critical effect?
• Describe other studies that support this finding.
• Discuss any valid studies which conflict with this finding.
As many relevant studies as possible should be collated and rigorously assessed as to their strengths and weaknesses to determine the key studies. This is particularly important where quantitative risk estimates will be undertaken or where there are apparently contradictory studies; in the latter case, the studies that are considered to be adequate in their design and interpretation will need to be appraised to determine the overall weight-of-evidence.
2. Besides the health effect observed in the key study, are there other health endpoints of concern?
• What are the significant data gaps?
3. Discuss available epidemiological or clinical data. For epidemiological studies:
• What types of studies were used, ie, ecologic, case-control, cohort?
• Describe the degree to which exposures were adequately described.
• Describe the degree to which confounding factors were adequately accounted for.
• Describe the degree to which other causal factors were excluded.
In assessing the relationship between a possible cause and an outcome, there must be a careful appraisal of whether the results could be explained by selection or measurement bias, confounding or chance (Beaglehole et al 1993). Particularly rigorous scrutiny should be given to studies giving a positive but not statistically significant result. A systematic and sequential approach to determining the nature of an association is detailed in ‘Guidelines for causation’ (Beaglehole et al 1993, p 76):
Temporal relation | Does the cause precede the effect (essential) |
Plausibility | Is the association consistent with other knowledge? (mechanism of action; evidence from experimental animals) |
Consistency | Have similar results been shown in other studies? |
Strength | What is the strength of the association between the cause and the effect? |
Dose-response relationship | Is increased exposure to the possible cause associated with increased effect? |
Reversibility | Does the removal of a possible cause lead to reduction of disease risk? |
Study design | Is the evidence based on a strong study design? |
Judging the evidence | How many lines of evidence lead to the conclusion? |
4. How much is known about how (ie through what biological mechanism) the chemical produces adverse effects?
• Discuss relevant studies of mechanisms- of- action or metabolism.
• Does this information aid in the interpretation of the toxicity data?
• What are the implications for potential health effects?
5. Comment on any non-positive (ie negative and equivocal) data in animals or people, and whether these data were considered in the hazard identification.
6. Summarise the hazard identification and discuss the significance of the following:
• confidence in conclusions;
• alternative conclusions that are also supported by the data;
• significant data gaps; and
• highlights of major assumptions.
7.2.2 Characterisation of Dose-Response
1. What data were used to develop the dose-response curve? Would the result have been significantly different if based on a different data set?
If animal data were used:
• Which species were used: most sensitive, average of all species, or other?
• Were any studies excluded? Why?
• If epidemiological data were used:
- Which studies were used: only positive studies, all studies, or some other combination?
- Were any studies excluded? Why?
- Was a meta-analysis performed to combine the epidemiological, studies? What approach was used? Were studies excluded? Why?
2. What model was used to develop the dose-response curve? What rationale supports this choice? Is chemical-specific information available to support this approach?
For non-carcinogenic hazards:
• How was the Tolerable Intake (or the acceptable range) calculated?;
• What assumptions or uncertainty factors were used?
• What is the confidence in the estimates?
• For carcinogenic hazards:
• What dose-response model was used? LMS, or other linear-at-low dose model, a biologically-based model based on metabolism data, or data about possible mechanisms of action?
• What is the basis for the selection of the particular dose-response model used? Are there other models that could have been used with equal plausibility and scientific validity? What is the basis for selection of the model used in this instance?
3. Discuss the route and level of exposure observed in the studies as compared to expected human exposures in site contamination situations.
• Are the available data from the same route of exposure as the expected human exposures? If not, are pharmacokinetic data available to extrapolate across routes of exposure?
• How far does one need to extrapolate from the observed data to environmental exposures (one to two orders of magnitude? multiple orders of magnitude)? What is the impact of such an extrapolation?
7.3 Mixtures
Currently there is no agreed Australian approach to assessing mixtures of contaminants. Where data (including mechanistic data) is available on the interaction of contaminants this should be taken into account in the assessment of a site. For more discussion on mixtures, see Schedule B(7A), Section 14.
A process for the appraisal of mixtures has been proposed as part of the future work plan that will arise from the Measure.
7.4 Sources of Toxicological Assessment Data
The following categories are given in order of preference. All documents, particularly those in the second and third categories require rigorous appraisal for relevance, validity and accuracy.
7.4.1 Principal Sources:
• World Health Organization (WHO) documents. Australia is a party to the WHO process and has incorporated their material in a variety of environmental health criteria. WHO documents include those from the International Programme on Chemical Safety such as the Environmental Health Criteria and documents detailing Tolerable Daily Intakes (TDI) or Tolerable Weekly Intakes (TWI) established by the Joint FAO/WHO Expert Committee on Food Additives (JECFA). This will be a source of TDIs, ADIs and PTWIs.
• National Health & Medical Research Council documents and documents from other joint Commonwealth, State and Territory health organisations. These may be a source of Australian guidance values.
• National Environmental Health Forum Documents distributed by the South Australian Health Commission for the Directors of Environmental Health.
• US Agency for Toxic Substances and Disease Registry documents for general toxicological reviews and Reference Doses
• National Toxicology Program carcinogenicity appraisals which document the results of carcinogenicity tests of a wide range of chemicals
• International Agency for Research on Cancer (IARC) documents
• NICNAS Priority Existing Chemical (PEC) reports
• IPCS Concise Information Chemical Assessment Documents (CICAD)
• OECD Standard Information Data Sets (SIDS)
7.4.2 Secondary Sources
7.4.2.1 Peer-reviewed journals
These may not provide opinions that meet general scientific agreement. With justification, and acceptance by the local jurisdiction, they may be suitable for use. Examples are:
• Risk Analysis
• Regulatory Toxicology and Pharmacology
• Human and Ecological Risk Assessment
• Food and Chemical Toxicology
• Carcinogenesis
• Environmental Health Perspectives
• Scandinavian Journal of Work, Environment and Health
• Journal of Occupational and Environmental Medicine
7.4.2.2 Industry Publications
With justification, and acceptance by the local jurisdiction, they may be suitable for use:
• European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC): Monographs, JACC Reports and Technical Reports
• Chemical Industry Institute of Toxicology (CIIT) reports
7.4.2.3 Occupational Health & Safety Sources
These may be a useful source for toxicological data and reviews but occupational exposure criteria must not be used in a general public health context without appropriate adjustment for the different durations of exposure, the inclusion of susceptible subpopulation in the general community (eg children) and the methodological differences in the setting of criteria.
7.4.3 Tertiary Sources
The use of this information requires justification that no other sources are available and an appraisal of the methodology detailing the level of conservatism and range of uncertainties inherent in the approach.
• US Integrated Risk Information System (IRIS) for cancer slope factors. (Australian health agencies have not established levels of "acceptable risk").
7.5 Methods for Risk Assessment of Carcinogens
The material in 7.5.1 and 7.5.2 is drawn, with amendment, from the NHMRC 'Draft Cancer Risk Assessment for Environmental Contaminants' (1997, pp1-16) which was prepared by a Technical Working Party (TWP). It has been reviewed and changed following a process of public consultation. The redrafted version is titled 'Toxicity Assessment Guidelines for Carcinogenic Soil Contaminants'. It was endorsed by NHMRC in September 1998.
The methodology was presented at the Fourth National Workshop on Health Risk Assessment and Management of Contaminated Sites held in Brisbane, October 1996 (Langley et al, 1998) and was considered by the Joint ANZECC/NHMRC Contaminated Sites Technical Review Committee also in October 1996. Both the
Workshop and the Joint ANZECC/NHMRC Contaminated Sites Technical Review Committee endorsed the approach taken by the TWP and supported the further development of the methodology.
This approach is consistent with other international risk assessment methodologies. The development and use of an agent-specific Guideline Dose is consistent with current risk assessment practice in Australia as well as with international practice. For example, the Guideline Dose and its use in risk assessment is analogous to the Acceptable Daily Intake (ADI) and the US Reference Dose (RfD). In Australia, due to variations in the circumstances of use and the nature of the chemicals being regulated, a range of different methodologies are used by different government agencies
7.5.1 Background
A variety of risk assessment methods has been used elsewhere, for example by the United States Environmental Protection Agency (US EPA, 1986), and the World Health Organisation (WHO, 1993).
As biological advances, mechanistic data, pharmacokinetic data and other relevant data are increasingly being taken into account in classifying and assessing the risks of carcinogens.
The TWP believed that existing methodologies had difficulties in conveying the health implications of exposure to environmental pollutants. The result in many cases has been an inequity of regulatory, political and public attention between cancer and other-than-cancer health effects.
The traditional benchmark dose methodology (traditional BMD) has been developed over the last two decades and is now being given serious consideration as a useful tool in risk assessment (Dourson, 1984; Barnes et al., 1995; US EPA, 1995a; 1996). More recently, the approach has also been proposed for cancer risk assessment (eg US EPA, 1996)
The TWP sought to develop a methodology for use in Australia which avoided some of the limitations inherent in existing cancer risk assessment methods. In particular, the methodology optimises the advantage of using all relevant scientific data in the decision-making process and provides for a clear separation and justification of the major components of the process: public health policy, professional judgement and scientific principles and data.
The methodology is a two step process. Firstly, the modified-BMD is derived from the experimental data. Secondly, the modified-BMD is divided by cumulative factors to derive a Guideline Dose for human exposure. The steps are outlined in Figure 7-I.
The modified-BMD is set using 5% extra risk determined from animal or epidemiological studies. After consideration of all the available toxicological data, this extra risk is then divided by a series of modifying factors (potentially up to 50,000) according to a specified decision tree to derive an agent specific Guideline
Dose protective of public health. These factors relate to inter- and intraspecies variation, quality of the data base and other factors for the seriousness of the carcinogenic response. The factors are derived using a decision tree which takes into account all of the available data; Scientific judgement is used to address a number of the uncertainties in the risk assessment process and in the development of safety factors.
The TWP supported the use of all available, relevant information in the risk assessment process. In cases where there are few or inadequate data, conservatism may be justified and the use of conservative (default) assumptions was supported. Recommendations on default assumptions are provided for cases where the data are incomplete to bridge data gaps and allow the risk assessment to proceed. All choices, both those based on scientific data and those based on default assumptions, must be supported by reasoned and critical analytical arguments.
The Guideline Dose is established by regulatory authorities and is defined as the daily intake of a chemical agent which, during a life time, is unlikely to result in cancer, based on a comprehensive expert assessment of the best information available at the time. It is considered that the Guideline Dose is protective of public health.
The Guideline Dose may be used in the development of health investigation levels, response levels and risk characterisation of human exposures to contaminants in soil.
The Guideline Dose does not attempt to model or predict a response incidence at low environmental exposure. It is an estimate of the dose which is considered protective of public health (the compounding use of factors assures a high level of safety). This places the focus of regulation on the control of exposure to environmental contaminants rather than calculation or discussions of risk. This approach has the added benefit of allowing comparisons with guidance values based on non-cancer health effects for chemical agents.
The TWP did not recommend a numerical value which would constitute an acceptable level of risk for low-level environmental exposure to carcinogens. Whilst there has been considerable debate over the last twenty years about what constitutes an acceptable risk, there is no agreed position internationally on this issue (see Department of the Environment, 1993).
Key points about the methodology are:
• Maximum use is made of scientific information, while not requiring the assessor to make a judgement regarding the existence of a biological threshold, nor perform mathematical dose-response modelling well below the range of experimental data because the dose associated with 5% extra risk is set near the lower limit of responses that can be measured experimentally. With the proposed methodology, it is not necessary to resolve the uncertainties, difficulties and controversies associated with mathematical extrapolation to low doses outside the range of experimental data.
• The approach is relatively model-independent when compared with methods which extrapolate to extremely low doses in the sense that the values of the
modified-BMD which are determined are not greatly influenced by the mathematical model chosen. Therefore, different models can be fitted to the data with similar goodness of fit. In contrast, extrapolation well below the experimental range by other quantitative risk assessment methods is very much model dependent and results are highly variable with different models (Maynard et al., 1995).
• The modified-BMD is standardised to one level of extra risk (ie. 5%), allowing comparisons of potency between carcinogens in the observed dose range in the animal bioassay or other modelled data. In addition, extra risk in the observed range can be compared between carcinogens for a given dose.
• The modified-BMD method is applied to both genotoxic or non-genotoxic carcinogens. In addition, it readily allows for the direct use of mechanistic data when an appropriate mechanistic model relating to dose-response can be developed. The TWP considered that the distinction between genotoxic and non-genotoxic features of carcinogens is relevant to public health protection and should be considered in the cancer risk assessment, but not in determining the shape of the dose-response curve at doses well below the experimental range. The genotoxic properties of an agent are an important part of the assessment and are accounted for in the consideration of the seriousness of the carcinogenic response.
• The modified-BMD is a numerical estimate of the dose associated with a particular response and by itself does not reflect the uncertainties inherent in biological data. Due care should be taken to describe the uncertainties (Lu and Sielken Jr, 1991).
The methodology can be compared to non-threshold models currently in use which assume low dose linearity (eg the US EPA methodology). The non-threshold models are inflexible and generally do not take account of the complexities of the events between exposure to an agent and the induction of a neoplasm. Risks estimated at doses below the range of experimental data can vary considerably depending on the model used, even though the various mathematical models used generally fit the experimental data equally well (Crump, 1984; Paustenbach, 1995). The numerical expression of the calculated level of risk falsely gives the impression that it represents an exact measure of actual risk. This numerical expression provides little or no information on the uncertainties related to the calculated level of risk, nor does it allow comparison with values for non-cancer health effects. Low-dose linearity assumes a positive slope of the dose-response curve at zero dose and implies that a single, irreversible genetic event at the initiation stage of carcinogenesis leading to transformation of a cell, is sufficient by itself to lead to the development of cancer. The major difficulty in this debate is the impossibility of testing experimentally the shape of the dose-response curve at extremely low doses (Purchase and Auton, 1995).
7.5.2 General principles of the methodology
1. Identify the relevant soil contaminants.
2. For each of the contaminants, check whether an ADI, PTWI or TDI has been set by the WHO. In cases where an ADI, PTWI or TDI is available, then:
• Ascertain whether there are new data which should be assessed or whether the derivation of the tolerable intake should be reviewed. If yes, proceed to step 3.
• If cancer or genotoxicity was not a consideration in deriving the value and current scientific information does not change the judgement that cancer should not be considered, then use the ADI, PTWI or TDI as described elsewhere for adverse effects other than cancer (ANZECC/NHMRC, 1992):
• If the carcinogenic or genotoxic properties of the chemical agent were assessed and considered in deriving the guidance value, the derivation of the guidance value (and any compelling new scientific evidence) should be reviewed and a decision made whether or not the toxicological properties of the substance should be reassessed. If yes, proceed to step 3. If no, then use the ADI, PTWI or TDI as described elsewhere for adverse effects other than cancer (ANZECC/NHMRC, 1992).
If no ADI, PTWI or TDI is available, proceed as follows: (Note: Appendices refer to NHMRC (1999))
3. Search the peer-reviewed scientific literature or any other, scientifically sound, available source to find all relevant data. Assess the adequacy of data collected to determine which will be selected for use in undertaking the following steps (Appendix A).
4. Based on studies judged to be adequate, determine whether the contaminant poses a carcinogenic hazard (Appendix B).
5. If the agent does not pose a carcinogenic hazard or if there is insufficient information currently available to make an assessment, no further evaluation of the carcinogenic hazard is needed. Proceed as for adverse effects other than cancer for development of a health-based regulatory value (see Addendum 1). Write findings in the report (Appendix G).
6. If the agent is considered to pose a carcinogenic hazard, determine whether the observed carcinogenic hazards are relevant to humans (Appendix C). If found to be not relevant, no further evaluation of the carcinogenic hazard is needed. Proceed as for adverse effects other than cancer for development of a health-based regulatory value (see Addendum 1). Write findings in the report (Appendix G).
7. If carcinogenic hazards are considered relevant to humans, apply the modified-BMD method and determine a modified-BMD for all relevant carcinogenic end-points corresponding to 5% and 1% extra risk (Section 2.4, figure 3 of NHMRC (in press) and Appendix D).
8. Use route to route extrapolation where appropriate (Appendix E).
9. Derive and apply appropriate factors to calculate Guideline Doses for each modified-BMD0.05 (Appendix F).
10. Choose the lowest Guideline Dose supported by the highest possible strength and weight of evidence.
11. Compare the Guideline Dose for the cancer end-point with the ADI, PTWI or to determine whether the carcinogenic end-point is the most sensitive one. Use the lowest of these doses for setting health investigation levels or for site specific risk assessment as outlined in the 'Guidelines for Assessment and Management of Contaminated Sites' (ANZECC/NHMRC, 1992).
12. Write the report (Appendix G).
Figure 7-I
Decision Tree for Cancer Risk Assessment

from NHMRC (1999)
Note: Appendices refer to NHMRC (1999)
7.5.3 Further actions
Guidelines are being developed because of deficiencies in current methodologies. Efforts are being made to develop and instigate guidelines and to provide Guideline Doses for specific chemicals. As an interim measure, advice on specific chemicals should be sought from the relevant regulatory body. When probabilistic estimates of risk are the only guidance available, there needs to be a full appreciation of the differences between ‘real’, ‘calculated’ and ‘perceived’ risk. The ‘real risk’ is the actual risk. The objective of risk assessment is to calculate as closely as possible the ‘real risk’. All participants in the process (different experts, different members of the community) will have different perceptions of the nature and magnitude of risk and these perceptions will change as circumstances change.
8. RISK CHARACTERISATION
8.1 Introduction
Risk characterisation is the final step in the risk assessment process that:
• integrates the individual characterisations from data collection, exposure assessment and toxicity assessment
• provides an evaluation of the overall quality of the assessment and the degree of confidence the authors have in the estimates of risk and conclusions drawn
• describes the risks to individuals and populations in terms of extent and severity of probable harm; and
• communicates results of the risk assessment to the risk manager (US EPA, 1995, p4)
The final risk characterisation is rarely accurately quantitative because of the limitations of the data which will be reflected in the uncertainty assessment. The process requires considerable expertise. If data are collected and analysed according to the principles and guidelines in this document the process will become more transparent and consistent. Some parts of the risk assessment process such as 'data collection' and 'exposure assessment' will be, at least in part, quantifiable. These guidelines are intended to assist the qualitative process of determining whether remediation is required or not for the proposed use. Due to the complexities of the matter, the risk characterisation process cannot be reduced to a 'cookbook'.
8.2 Key Principles in Health Risk Characterisation
There are a number of principles which form the basis for a risk characterisation:
Risk assessments should be transparent, in that the conclusions drawn from the science are identified separately from policy judgements, and the use of default values or-methods and the use of assumptions in the risk assessment are clearly articulated.
• Risk characterisations should include a summary of the key issues and conclusions of each of the other components of the risk assessment, as well as
describe the likelihood of harm. The summary should include a description of the overall strengths and the limitations (including uncertainties) of the assessment and conclusions.
• Risk characterisations (and risk assessments) should be consistent in general format, but recognise the unique characteristics of each specific situation.
• Risk characterisation is a key component of risk communication, which is an interactive process involving exchange of information and expert opinion among individuals, groups and institutions. (US EPA 1995)
• The primary aims of risk assessment are to protect public health and the environment, putting these responsibilities before all other considerations. Health risk assessment must be undertaken with an appreciation that the health risk assessment is part of a larger assessment that encompasses ecological risk assessment.
• To protect public health and the environment an appropriate degree of conservatism must be adopted to guard against uncertainties.
• Ensure that comparisons of contaminant levels have been made with the National Environmental Health Forum Monographs on 'Health-based Investigation Levels' and 'Exposure Scenarios and Exposure Settings' and, for groundwater, the NHMRC/ARMCANZ (1996) Australian Drinking Water Guidelines. For occupational health risk assessment of airborne contaminants, the NOHSC occupational exposure standards for airborne contaminants should be used.
• Where there are no Health-based Investigation Levels for a particular substance refer to the administrative authority for contaminated sites in the relevant State or Territory
• Where necessary, undertake health risk assessments according to methods in the NEPM ‘Assessment of Site Contamination’ and associated guidelines, ANZECC/NHMRC ‘Australian and New Zealand Guidelines for the Assessment and Management of Contaminated Sites’ (1992), national and/or international toxicological assessments (such as WHO, NHMRC) or, where these are unavailable, using methods approved by the administrative authority for contaminated sites in the relevant State or Territory.
• When deriving soil or groundwater criteria use toxicological data or exposure criteria from agencies or organisations relevant to the State or Territory (eg local or Commonwealth health agencies such as NHMRC, or the National Environmental Health Forum) or to which Australia is party (eg World Health Organization).
• Site-specific risk assessors should maintain up-to-date knowledge of the peer-reviewed scientific literature relevant to risk assessment.
(dotpoints 5-10 adapted from EPA NSW, 1998)
8.3 Conceptual Guide for Developing Chemical-Specific Risk Characterisations
US EPA (1995) provides a common format to assist risk managers in evaluating and using risk characterisation.
The outline has two parts. The first part requires summaries of the major components of the risk assessment leading up to the risk characterisation.
The second part draws all of the information together to characterise risk. The outline described below represents the expected findings for a typical complete chemical assessment for a single chemical. Exceptions due to the circumstances of individual assessments such as particular statutory requirements, resource limitations, and other specific factors should be explained as part of the risk characterisation.
Minor variations in its application from one instance to another are appropriate and expected and are not a legitimate basis for delaying or complicating action on otherwise satisfactory scientific, technical, and regulatory products. (US EPA, 1995)
8.4 Risk Conclusions
A modified version of the US EPA (1995) framework for risk conclusions is a useful model to be used in Australia
8.4.1 Risk Conclusions
1. What is the overall picture of risk, based on data collection, exposure assessment, toxicity assessment and risk characterisation?
2. What are the major conclusions and strengths of the assessment in each of the three main analyses (ie., hazard identification, dose-response, and exposure assessment)?
3. What are the major limitations and uncertainties in the three main analyses?
4. What are the science policy options in each of the three major analyses?
5. What alternative approaches have been evaluated?
6. What are the reasons for the choices made?
8.4.2 Risk Context
1. What are the qualitative characteristics of the hazard: (eg, voluntary vs. involuntary, technological vs. natural, etc.)? Comment on findings, if any, from studies of risk perception that relate to this hazard or similar hazards.
2. What are the alternatives to this hazard? How do the risks compare?
3. How does this risk compare to other risks?
• How does this risk compare to other risks in this regulatory program, or other similar risks that the regulatory agencies have made decisions about?
• Where appropriate can this risk be compared with past regulatory agency decisions or common risks with which people may be familiar?
• Describe the limitations of making these comparisons.
4. Comment on significant community concerns which influence public perception of risk?
8.4.3 Existing Risk Information
Comment on other risk assessments that have been done on this chemical by State, Territory or federal agencies, or other organisations. Are there significantly different conclusions that merit discussion?
8.4.4 Other Information
Is there other information that would be useful to the risk manager, or the public in this situation that has not been described above?
8.5 Uncertainty
Uncertainty analysis must be addressed for each step of the risk assessment and for its cumulative effect from all of the steps.
The assessment of uncertainty is a critical part of the risk assessment process. Uncertainty characterisation is an essentially qualitative process relating to the selection and rejection of specific data, estimates, scenarios, etc (US EPA, 1992). Uncertainty assessment can be more quantitative and it may be represented by more simple measures such as ranges, simple analytical methods such as sensitivity analysis and may progress to complex measures and techniques (Langley, 1993).
Uncertainty (ie. the lack of knowledge about the correct value, for example a specific exposure measure or estimate) must be distinguished from variability (ie. different levels of exposure experienced by different individuals).
Uncertainty may need to be addressed by the collection of further data.
Further detail on uncertainty analysis is available in Vic EPA (1997a).
Table 8-A gives an example of an uncertainty table.
Table 8-A
Example of an uncertainty table for exposure assessment
| Effect on Exposure2 | ||
Assumption | Potential Magnitude for Over-Estimation of Exposure | Potential Magnitude for Under-Estimation of Exposure | Potential Magnitude for Over- or Under-Estimation of Exposure |
Environmental Sampling and Analysis |
|
|
|
Sufficient samples may not have been taken to characterise the media being evaluated, especially with respect to currently available soil data. |
|
| Moderate |
Systematic or random errors in the chemical analyses may yield erroneous data. |
|
| Low |
Exposure Parameter Estimation |
|
|
|
The standard assumptions regarding body weight, period exposed, life expectancy, population characteristics, and lifestyle may not be representative of any actual exposure situation. |
|
| Moderate |
The amount of media intake is assumed to be constant and representative of the exposed population. | Moderate |
|
|
Assumption of daily lifetime exposure for residents. | Moderate to High |
|
|
Use of "hot spot" soil data for upper-bound lifetime exposure | Moderate to High |
|
|
![]() 2 As a general guideline, assumptions marked as "low", may affect estimates of exposure by less than one order of magnitude; assumptions marked "moderate" may affect estimates of exposure by between one and two orders of magnitude; and assumptions marked "high" may affect estimates of exposure by more than two orders of magnitude. (adapted from US EPA, 1989a, p6-51)
2 As a general guideline, assumptions marked as "low", may affect estimates of exposure by less than one order of magnitude; assumptions marked "moderate" may affect estimates of exposure by between one and two orders of magnitude; and assumptions marked "high" may affect estimates of exposure by more than two orders of magnitude. (adapted from US EPA, 1989a, p6-51)
Reasons for addressing uncertainties in assessments include (US EPA, 1992 with slight amendment):
• The combination of uncertain information from various sources.
• Having to make decisions about whether further resources should be expended on seeking further information and data to reduce uncertainty.
• As a means of highlighting biases that may have crept into the process.
• As assessment is an iterative process, uncertainty analysis may enhance the outcome of the process.
• Risk assessment may be one of several processes involved in a particular situation. Being able to characterise the uncertainty will assist the decision-makers and ultimately improve the decision making.
• "Risk assessors have a responsibility to present not just numbers but also a clear and explicit explanation of the implications and limitations of their analyses. Uncertainty characterisation helps carry out this responsibility.
8.6 Exceedances of Acceptable Daily Intakes (ADIs)
Renwick and Walker (1993, p 464) provide two excerpts from WHO documents relating to exceedances of ADIs:
'Because in most cases, data are extrapolated from life-time animal studies, the ADI relates to life-time use and provides a margin of safety large enough for toxicologists not to be particularly concerned about short-term use at exposure levels exceeding the ADI, providing the average intake over longer periods does not exceed it.' (WHO, 1987)
Further information was provided in a report in 1989 which added that:
It is impossible to make generalisations concerning the length of time during which intakes in excess of the PTWI (provisional tolerable weekly intake) would be toxicologically detrimental.
Any detrimental effect would depend upon the nature of the toxicity and the biological half-life of the chemical concerned.' (WHO, 1989)
The following discussion on the significance of exceeding the ADI applies equally to other recommended limits of intake or exposure, such as TDI or PTWI.
Renwick and Walker (1993) propose three questions that need to be considered if there are potential exceedances of the ADI.
• What proportion of the population should be allowed to exceed the ADI?
• To what extent can the ADI be exceeded without any real concern?
• How long does the person need to exceed the ADI before there is a cause for real concern?
(Renwick and Walker, 1993, p 464)
They stress 'the significance of any minor excursions of intake above the ADI can only be put into context by reference back to the animal data and to the NOEL which gave rise to the ADI' (p 465).
Renwick and Walker describe three parameters governing the precision of the No Observed Effect Level:
• the sensitivity of the toxicological end point which depends on the incidence of the lesion in control animals and/or its inter-animal variability;
• the group size studied which tends to be less important than;
• the increment between doses. There may be considerable increments between doses and this can result in a no observed adverse level that can be significantly lower than the actual or absolute No Observed Effect Level.
Renwick and Walker (1993) conclude that the significance of the exceedance must be assessed on a substance-specific basis and by reference to the toxicological (and especially the NOEL) data; the magnitude of the exceedance, and the duration of the exceedance. (Langley and Sabordo 1996, p144)
9. APPRAISAL OF ASSESSMENTS
Langley (1993a) presents methods for appraising assessments.
9.1 Introduction
Accurate and timely site-specific health risk assessments will depend upon coherent and logically developed reports; See Schedule B(2) for standardised formats for data Collection and reporting. Regulatory agencies should immediately reject reports that:
• are unclear and confusing
• do not meet appropriate levels of coherence or logic
• do not meet the requirements of the standardised formats or the checklist of health risk assessment contents.
9.2 General
A person preparing or reviewing an assessment will consider questions such as:
9.2.1 Key Aspects
• Has the objective of the report been defined clearly?
• Is there a clear understanding of the proposed landuse and whether any constraints (eg encumbrances) will be acceptable?
•
• Was the sampling reasonably sufficient to identify, to locate and to demarcate any potential contamination?
• Is it clear how results of any sampling plan were analysed and interpreted?
• Have data been analysed en masse or for the appropriate strata?
• Were environmental fate and transport mechanisms understood?
• Have the data been 'modelled' to demonstrate a three dimensional understanding of what is occurring on the site?
• How were abnormal results or findings managed?
• Were the uncertainties of the assessment identified and understood?
9.2.2 Interpretation of Data
An appraisal of data must show an understanding of:
• the site history (and gaps in the history)
• topography of the site
• soil structure (eg presence of clay or fill, and the depths of individual strata)
• the proposed land use
Too often numerical data are considered in isolation from other key parameters such as:
• soil characteristics (eg from what stratum did the sample come)
• the levels of detection (and reporting)
• the geographical relationship of one sample to another
• the proposed landuse
Other key failings in the analysis of numerical data include:
• Ignoring negative or unexceptional results by focussing on unusual or elevated results: the data set needs to be considered in its entirety.
• Inadequately managing censored data eg by assigning a zero value to results below the level of detection or reporting
• Accepting relatively high levels of detection or reporting so that the value of much data is obscured. This may have the consequence of failing to reveal gradients that will help to highlight the presence and location of 'hot spots'. Examples have been seen where Dutch 'B' levels have been treated as the level of reporting. The very existence of levels of detection and reporting result in the censoring of data. Censoring of data can be particularly important when the maximum permitted criterion is close to the level of detection (eg with potable drinking water standards). The censoring of data must be addressed in an appropriate way (see Heyworth, 1991).
Given two similar results, the result that can be explained (eg by history, or similarities with results from similar strata) will tend to be of less concern.
9.2.3 Use of Subjective Terms
The use of subjective terms in reports (eg 'heavy/medium/light contamination') or terms that are used in common parlance but may have legalistic definitions (eg 'contamination') is confusing and should be avoided in reports. The use of the term 'hot spot' can be rather misleading to the general public and is best avoided.
9.3 Specific
9.3.1 Human health risk assessment checklist
The following checklist has been adapted from EPA NSW (1998) and Vic EPA (1997a) and should be addressed in human health risk assessments.
9.3.1.1 Data Collection
• Have the objectives of the risk assessment been stated?
• Has the background to the events leading to the risk assessment been provided?
• Have all chemicals of potential concern been identified and appraised?
• Have all appropriate sources of information regarding chemicals of potential concern been identified and appraised?
• Has justification been given for the selection of the chemicals of potential concern? Has justification been given for the omission of chemicals from the analysis?
• Have the sources of the contaminants been identified?
• Have the environmental fate and transport of the contaminants been identified?
9.3.1.2 Toxicological Assessment
• Have all relevant toxicological facts been checked for accuracy and currency?
• Has the adequacy of the available toxicological database been appraised?
• Have the effects on each significant body system (for example, renal, hepatic, cardiovascular,) and the types of effects (for example, allergy, genotoxicity and carcinogenicity, reproductive and developmental) been appraised and summarised for the relevant exposure routes?
• Has the critical toxic effect(s) and organ/body system been identified?
• Have known toxicity modifying factors (such as synergistic and antagonistic effects resulting from exposure to multiple contaminants) been considered?
• Have toxicologically sensitive subpopulations been identified?
• Has the toxicological basis of the guidance value or potency factor, where applicable, been discussed and the uncertainties noted?
• Have NHMRC (where applicable) or WHO toxicological assessments been considered as the primary toxicological resource?
• Where relevant, have differences between, for example, WHO and US EPA toxicological assessments been appraised and discussed?
• Has the dose-response relationship for chemicals of potential concern been appraised and discussed?
• Have the data been presented in a form amenable to efficient interpretation and review?
9.3.1.3 Exposure Assessment
• Has the potentially exposed population been identified?
• Have potentially exposed, unusually susceptible sub-populations been identified?
• Have the estimates of chemical exposure for each significant exposure route and for each chemical of potential concern been adequately quantified and tabulated?
• In cases of presumed insignificant exposure, has the exposure been demonstrated to be small?
• Has the relative significance of each exposure pathway, based on the risk analysis, been discussed?
9.3.1.4 Equations
• Have all equations used in the risk assessment been presented in the report?
• Are all equations consistent?
• Have all parameters in each equation been clearly defined?
• Have the correct units been allocated to each parameter?
• Are all equations dimensionally correct?
• Have all unit conversion factors, where applicable, been included in the equations?
• Has all pertinent information been provided to enable calculations to be checked through in a stepwise process?
9.3.1.5 Data Evaluation
• What were the data collection objectives and are they consistent with the requirements of the risk assessment?
• Have the laboratories that did the chemical analyses been noted, and do they have NATA, or equivalent, accreditation to perform the chemical analyses?
• Has laboratory QA/QC been reported and analysed?
• Has field QA/QC been reported and analysed?
• Where appropriate, has the size of the 'hot spot' detectable by the sampling pattern been stated?
• Have statements of the accuracy of the laboratory data for each contaminant been made?
9.3.1.6 Assessment and report presentation
• Have all tables and figures been referred to correctly in the text of the report?
• Has irrelevant information from other sites been excluded from the report?
• Has information from previous reports on the site been appropriately selected and incorporated into this report?
• Have all assumptions and default data been identified and justified?
• Has the analysis been based on an up-to-date literature appraisal?
• Have all conclusions been justified?
• If toxicological data and the exposure scenario lead to the conclusion that a high concentration of contaminant is permissible in terms of human health, does the result violate ecological, aesthetic, land-use or physical principles?
• Has a risk management decision(s) been made during the course of the risk assessment and, if so, how might it (they) have influenced the calculation of risk?
• Has a detailed uncertainty discussion been included in the report?
• Has information been presented coherently and in an appropriate sequence, to enable efficient appraisal of the report?
• Does the report include or enable ecological risk assessment as required by regulatory authorities?
10. USE OF HEALTH RISK ASSESSMENT TO DEVELOP HEALTH-BASED SOIL CRITERIA
The following method was used for developing Health-based Investigation Levels. Similar principles have been used for determining HILs for contaminants with and without cancer endpoints. The methodology was initially endorsed in ANZECC/NHMRC(1992). This approach can also be used for developing Response Levels by using site-specific data and appropriate safety factors to accommodate uncertainty and variability. Site-specific Response Levels developed by risk assessors will require endorsement by regulators and/or auditors. Guideline Doses for carcinogenic soil contaminants will be established by regulatory authorities.
Investigation levels will be determined taking into account (ANZECC/NHMRC 1992, Imray and Langley 1996):
• The bioavailability of a substance. The bioavailability should be assumed to be 100% if specific information is not available;
• The Provisional Tolerable Weekly Intake (PTWI) or Acceptable Daily Intake (ADI) as determined by the World Health Organization/Food and Agriculture Organization (1987, 1994), or Guideline Dose (GD) for cancer toxic effects as determined by national health advisory bodies;
• Other potential sources of the substances that comprise a proportion of the PTWI or ADI, or GD (eg. background levels of the substance in food, water, air and the amount of exposure through these routes). (WHO/FAO, 1987)
The total exposure to a substance 'X' can be represented by the equation:
Exposure to substance X = Background Exposures (eg. from food and water)
+
Exposures from contaminated soil by ingestion,
inhalation and skin absorption)
= Background Exposures
+
Amount of substance absorbed from soil.
= BE
+
(Sing x Cing x Bing + Sinh x Cinh x Binh + Sskin x Cskin x Bskin)
= BE + SEsoil
BE = Background Exposures (eg. from food and water).
Sing = Amount of soil ingested.
Sinh = Amount of soil/dust inhaled and retained.
Sskin = Amount of soil on skin.
Cing = Concentration of substance in soil ingested.
Cinh = Concentration of substance in soil/dust inhaled and retained.
Cskin = Concentration of substance in soil on skin.
Bing = Bioavailability, ie. percentage absorbed, of substance when ingested.
Binh = Bioavailability of substance when inhaled.
Bskin = Bioavailability of substance when on skin.
SEsoil = Substance exposure from soil."
(ANZECC/NHMRC, 1992, p37)
Different levels of bioavailability will occur between soil ingested, inhaled or in contact with skin.
National health investigation level guidelines will be set by national health advisory bodies.
A variable percentage of the TI will be allowed for exposure to contaminated soil. The variation will largely relate to contributions of other background factors, especially food. This is consistent with the IPCS approach and that used in the four Australian workshops on the health risk assessment and management of contaminated sites.
When the PTWI/ADI is used for establishing investigation levels for individual contaminants, the basis for the PTWI/ADI should be sought from appropriate World Health Organization documents (eg. WHO 1987, WHO 1989). This information should include target organ(s) and effect(s) (eg. nature, reversibility, severity, LOAEL for most significant toxic effect); bioavailability; and safety factors accounting for variations in human sensitivity and extrapolations from animal studies. Similarly, when a Guideline Dose derived using the NHMRC 'Toxicity Assessment Guidelines for Carcinogenic Soil Contaminants' is used, the basis for the derivation should be fully documented. Guideline Doses for soil contaminants with cancer effects will be determined by national health advisory bodies or their appointees.
If no PTWI, ADI, or GD is available a specific approach acceptable to the relevant health agencies will need to be determined using WHO (1994) for non carcinogens, or National Environmental Health Forum Guidance for Cancer Risk Assessment for substances with cancer effects.
It is considered that these methods for determining investigation levels will protect the entire population with few exceptions. Where a significant proportion of the population demonstrates allergic sensitisation to a substance (eg nickel) this will need to be considered in criteria setting. People who may have unusual sensitivity to contaminants may need to be considered in a site assessment (Imray and Langley, 1996).
Qualifications to setting the Health-based Investigation Levels are:
• In setting an investigation level guideline, total exposure to substance X, (ie. the sum of the background exposure and the substance exposure from soil) should not exceed the ADI or PTWI, (or GD) ie., BE+SEsoil < ADI or PTWI, (or GD).
• The degree to which exposures at a proposed investigation level guideline are below the ADI or PTWI, or GD will be set by national health advisory bodies and will depend on factors such as: the nature of the adverse effects, the completeness of toxicological data, exposure variability within a population and the relative sizes of BE and SEsoil.
• It should be recognised that '...short-term exposure to levels exceeding the PTWI is not a cause for concern provided the individual's intake averaged over longer periods of time does not exceed the level set' (WHO, 1989, p9).
A decision tree detailing the use of Health Risk Assessment to develop health-based soil criteria is provided in Figure 10-I.
11. REFERENCES
Aitio A, Jarvisalo J, Riihimaki V and Hernberg S (1988). 'Biologic monitoring'. In: Zenz C, ed. Occupational medicine - Principles and practical applications. Chicago: Year Book Medical Publishers.
Alsop WR, Ruffle B, Frohmberg EJ and Anderson PD (1993). 'Development of risk-based clean-up standards using Monte Carlo analysis' In: Developing clean-up standards for contaminated soil, sediment, and groundwater. How clean is clean? Washington: ATSDR/US EPA.
American Industrial Health Council Task Force (1994). Exposure Factors Sourcebook Washington: American Industrial Health Council.
Anderssen B and Markey B (1996). 'Volatilisation from soil and exposure assessment'. In: Langley A, Markey B and Hill H (eds) The Health Risk Assessment and Management of Contaminated Sites, Proceedings of the Third National Workshop on the Health Risk Assessment and Management of Contaminated Sites. Contaminated Sites Monograph Series Number 5. South Australian Health Commission, pp 441-468.
Anderssen B and Markey B (1997). Indoor infiltration of volatile organic contaminants: Coupling above - and below - ground via a non-homogeneous surface boundary condition. J. Soil Contamination. 6, 15-19.
Anderssen B and Markey B (1998). 'Exposure to volatiles from below-ground sources'. In: Langley A, Imray P, Lock W and Hill H (eds). The Health Risk Assessment and Management of Contaminated Sites, Proceedings of the Fourth National Workshop on the Health Risk Assessment and Management of Contaminated Sites. Contaminated Sites Monograph Series Number 7. South Australian Health Commission, pp 93-105.
Anderssen RS, de Hoog FR and Markey BR (1997). Modelling the volatilization of organic soil contaminants: Extension of the Jury, Spender and Farmer behaviour assessment model and solution. Appl. Math. Lett. 10(1), 31-34, and Erratum Appl. Math Lett. 10(6), 135.
ANZECC/NHMRC (1992). Australian and New Zealand Guidelines for the Assessment and Management of Contaminated Sites. Australian and New Zealand Environment and Conservation Council/National Health and Medical Research Council, Canberra.
ANZECC/NHMRC (1997) Draft. The assessment and management of contaminated sites. Policy Framework. Canberra: ANZECC/NHMRC.
Barnes et al (1995). Benchmark dose workshhop: criteria for use of a benchmark dose to estimate a reference dose. Regulatory Toxicology and Pharmacology. 21: 296-306.
Beaglehole R, Bonita R and Kjellstrom T (1993). Basic epidemiology. World Health Organization: Geneva.
Burmaster DE and Anderson PD (1994). Principles of Good Practice for the Use of Monte Carlo Techniques in Human Health and Ecological Risk Assessments. Risk Analysis, 14(4): 477-481.
Butterworth BE (1990). Consideration of both genotoxic and non-genotoxic mechanisms in predicting carcinogenic potential. Mutation Research 239: 117-132.
Calabrese EJ and Kostecki PT (1992). Risk assessment and environmental fate methodologies. Boca Raton: Lewis Publishers.
Crump K (1984). A new method for determining allowable daily intakes. Fundamental and Applied Toxicology 4: 854-891.
Department of the Environment (UK) (1993). Contaminants in soil: collation of toxicological data and intake values for humans. Project Number 7/10/333.
DOH (1991). Guidelines for the evaluation of chemicals for carcinogenicity. Committee on carcinogenicity of chemicals in food, consumer products and the environment. Department of Health, Report RHSS 42, HMSO London.
Dourson ML (1984). 'Acceptable Daily Intake (and discussion).' In: Approaches to risk assessment for multiple chemical exposures. Proceedings of the Workshop. September 29-30, 1982. EPA-600/9-84-008. Washington: US Environmental Protection Agency. 1-42.
European Chemical Industry Ecology and Toxicology Centre (ECETOC) (1990). Technical Report No. 40. Hazard Assessment of Chemical Contaminants in Soil. Brussels: European Chemical Industry Ecology and Toxicology Centre.
EPA NSW (1997). Contaminated Sites. Guidelines for consultants reporting on contaminated sites. Sydney, NSW Environment Protection Authority.
EPA NSW (1998). Draft Guidelines for the NSW Site Auditor Scheme. Sydney, Environment Protection Authority
Ferguson CC (1994). The Contaminated Land Exposure Assessment Model (CLEA): Technical Basis and Algorithms. Draft report submitted to Department of the Environment. Nottingham: The Nottingham Trent University Centre for Research into the Built Environment.
Finley B et al (1994). Recommended Distributions for Exposure Factors Frequently Used in Health Risk Assessment. Risk Analysis 14(4): 533-53.
Friberg LT (1985). The rationale of biological monitoring of chemicals - with special reference to metals. American Industrial Hygiene Association Journal 46: 633-641.
Hawley JK (1985). Assessment of health risks from exposure to contaminated soil. Risk Analysis, 5(4): 289-302.
Heyworth JS (1991). 'Sampling and statistical analysis for assessing contaminated land sites.' In: El Saadi O, Langley AJ (eds), The Health Risk Assessment and Management of Contaminated Sites Proceedings of a National Workshop on the Health Risk Assessment and Management of Contaminated Sites. Adelaide: South Australian Health Commission.
Imray P and Langley AJ, (1996). Health-based Investigation Levels. National Environmental Health Forum Monographs Soil Series No. 1. Adelaide: South Australian Health Commission.
IRIS (1996). Integrated Risk Information System. US Environmental Protection Agency, Washington DC.
Langley AJ (1991). 'Exposure Pathways.' In: El Saadi O and Langley AJ (eds), The Health Risk Assessment and Management of Contaminated Sites Proceedings of a National Workshop on the Health Risk Assessment and Management of Contaminated Sites. Adelaide: South Australian Health Commission, 1991.
Langley AJ (1991a). 'Protecting the Public: Biological Monitoring.' In: El Saadi O and Langley AJ (eds), The Health Risk Assessment and Management of Contaminated Sites Proceedings of a National Workshop on the Health Risk Assessment and Management of Contaminated Sites. Adelaide: South Australian Health Commission.
Langley AJ (1993). 'Refining exposure assessment.' In: Langley AJ, Van Alphen M. (eds), The Health Risk Assessment and Management of Contaminated Sites. Proceedings of the Second National Workshop on the Health Risk Assessment and Management of Contaminated Sites. Contaminated Sites Monograph Series No. 2. Canberra.
Langley AJ (1993a) 'The representation of data and the appraisal of contaminated land.' In: Langley A J, van Alphen M (eds), The Health Risk Assessment and Management of Contaminated Sites. Proceedings of the Second National Workshop. Adelaide: South Australian Health Commission.
Langley AJ and El Saadi O. (1991) Protocol for the Health Risk Assessment and Management of Contaminated Sites. Adelaide: South Australian Health Commission.
Langley AJ and Sabordo L (1996). 'Exposure Factors in Risk Assessment.' In: Langley A, Markey B and Hill H (eds) The Health Risk Assessment and Management of Contaminated Sites, Proceedings of the Third National Workshop on the Health Risk Assessment and Management of Contaminated Sites. Contaminated Sites Monograph Series Number 5. South Australian Health Commission, pp 137-190.
Langley AJ, Taylor A and Dal Grande E (1998). '1996 Australian Exposure Factors.' In: Langley A, Imray P, Lock W and Hill H (eds). The Health Risk Assessment and Management of Contaminated Sites, Proceedings of the Fourth National Workshop on the Health Risk Assessment and Management of Contaminated Sites. Contaminated
Sites Monograph Series Number 7. South Australian Health Commission, pp 137-190.
Lock W H. (1996) Composite sampling. National Environmental Health Monographs. Soil Series No. 3. Adelaide: South Australian Health Commission.
Lu FC and Sielken Jr RL (1991). Assessment of safety/risk of chemicals: inception and evolution of the ADI and dose-response modelling procedures. Toxicology Letters 59: 5-40.
Maynard RL, Cameron KM, Fielder R, McDonald A and Wadge A (1995). Setting air quality standards for carcinogens: an alternative to mathematical quantitative risk assessment - discussion paper. Human and Experimental Toxicology 14: 175-186.
McKone TE (1994). Uncertainty in Variability in Human Exposures to Soil Contaminants Through Home Grown Food: A Monte Carlo Assessment. Risk Analysis, 14(4): 449-463.
Mueller PW, Smith SJ, Steinberg KK, Thun MJ (1989). Chronic renal tubular effects in relation to urine cadmium levels. Nephron ; 52: 45-54.
National Academy of Sciences (1983). Risk Assessment in the Federal Government: Managing the process. Washington: National Academy Press.
National Health & Medical Research Council (NHMRC) (1987). Report of the one hundred and third session. June 1987. National Health and Medical Research Council, p.30.
NHMRC (1997) Draft Cancer Risk Assessment for Environmental Contaminants. Canberra: National Health & Medical Research Council. This document was released for public comment. The revised draft has been retitled Toxicity Assessment Guidelines for Carcinogenic Soil Contaminants
NHMRC (1999) Toxicity Assessment Guidelines for Carcinogenic Soil Contaminants Canberra: National Health & Medical Research Council. (previously titled Draft Cancer Risk Assessment for Environmental Contaminants.
Paustenbach DJ (1989). 'A survey of health risk assessment.' In: Paustenbach DJ (ed). The risk assessment of environmental and human health hazards: a textbook of case studies. New York: John Wiley & Son.
Paustenbach DJ (1995). The practice of health risk assessment in the United States (1975-1995): How the US and other countries can benefit from that experience. Human and Ecological Risk Assessment, 1: 29-79.
Purchase IFH and Auton TR (1995). Thresholds in chemical carcinogenesis. Regulatory Toxicology and Pharmacology 22: 199-205.
Renwick AG, Walker R (1993). An analysis of the risk of exceeding acceptable or tolerable daily intake. Regulatory Toxicologically and Pharmacology, 18: 463-480.
Ruffle B, Burmaster DE, Anderson PD, Gordon HD (1994). Lognormal distributions for fish consumption by the general US population. Risk Analysis, 14(4): 395-404.
Smith RL (1994). Monte Carlo simulation for human exposure assessment at a Superfund site. Risk Analysis, 14(4): 433-439.
Taylor R and Langley AJ (1998). Exposure scenarios and exposure settings. National Environmental Health Forum Monographs Soil Series No. 2. 2nd ed. Adelaide: South Australian Health Commission.
US EPA (1986). The risk assessment guidelines of 1986. Washington DC: Office of Health and Environmental Assessment, EPA/600/8-87/045, United States Environmental Protection Agency, Washington DC.
US EPA (1989). Risk assessment guidance for Superfund, Volume 1. Human health evaluation manual, (Part A). Interim final. EPA/540/1-89/002. Washington: US Environmental Protection Agency, Office of Emergency and Remedial Response.
US EPA (1989a). Exposure Factors Handbook. EPA 600/8-89/043. Washington: US Environmental Protection Agency.
US EPA (1992). Guidelines for Exposure Assessment; notice. Part VI. Federal Register 29 May 1992 57(104): 22888-22938.
US EPA (1992a). Guidance on risk characterisation for risk managers. Internal Memorandum by F.Henry Habicht. Washington; US Environmental Protection agency, Office of the Administrator.
US EPA (1995). Guidance for risk characterization. Washington: US Environmental Protection Agency Science Policy Council.
US EPA (1995a). The use of the benchmark dose approach in health risk assessment. Risk Assessment Forum, EPA/630/R-94-007, United States Environmental Protection Agency, Washington DC.
US EPA (1996). Draft Revision to the guidelines for carcinogenic risk assessment. Office of Health and Environmental Assessment, Office of Research and Development, United States Environmental Protection Agency, Washington DC
Vic EPA (1997a) National framework for ecological risk assessment of contaminated sites. Part A. Canberra: Environment Australia. (Victorian Environment Protection Authority)
WHO (1972). Evaluation of certain food additives and the contaminants mercury, lead, and cadmium. Sixteenth report of the joint FAO/WHO Expert Committee on Food Additives (JECFA). World Health Organization Technical Report Series 505. World Health Organization, Geneva.
WHO (1986). Early detection of occupational diseases. Geneva: World Health Organization, Geneva.
WHO (1987). Principles for the Safety Assessment of Food Additives and Contaminants in Food. Environmental Health Criteria, 70. World Health Organization, Geneva.
WHO (1989). Evaluation of certain food additives and contaminants. Thirty third report of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). World Health Organization Technical Report Series 776. Geneva; World Health Organization.
WHO (1989a). Guidelines for predicting dietary intake of pesticide residues. Joint UNEP/FAO/WHO Food Contamination Monitoring Programme. Geneva, World Health Organization.
World Health Organization (1993). Environmental Health Criteria 155. Biomarkers and risk assessment: concepts and principles.. International Programme on Chemical Safety (IPCS). Geneva; World Health Organization.
WHO (1993a). Guidelines for drinking water quality. Volume 1 Recommendations. Second Edition, World Health Organization, Geneva.
WHO (1994). Assessing human health risks of chemicals: Derivation of guidance values for health-based exposure limits. Environmental Health Criteria 170. International Programme on Chemical Safety (IPCS). Geneva; World Health Organization.