
![]()
![]()


![]()
![]()

 Page
Page
1 DDT+DDE+DDD Review
1.1 General
1.2 Previous HIL
1.3 Significance of Exposure Pathways
1.3.1 Oral Bioavailability
1.3.2 Dermal absorption
1.3.3 Inhalation of Dust
1.3.4 Plant Uptake
1.3.5 Intakes from Other Sources – Background
1.4 Identification of Toxicity Reference Values
1.4.1 Classification
1.4.2 Review of Available Values/Information
1.4.3 Recommendation
1.5 Calculated HILs
1.6 References
2 Aldrin + Dieldrin
2.1 General
2.2 Previous HIL
2.3 Significance of Exposure Pathways
2.3.1 Oral Bioavailability
2.3.2 Dermal absorption
2.3.3 Inhalation of Dust
2.3.4 Plant Uptake
2.3.5 Intakes from Other Sources – Background
2.4 Identification of Toxicity Reference Values
2.4.1 Classification
2.4.2 Review of Available Values/Information
2.4.3 Recommendation
2.5 Calculated HILs
2.6 References
3 Chlordane (total)
3.1 General
3.2 Previous HIL
3.3 Significance of Exposure Pathways
3.3.1 Oral Bioavailability
3.3.2 Dermal absorption
3.3.3 Inhalation of Dust
3.3.4 Plant Uptake
3.3.5 Intakes from Other Sources – Background
3.4 Identification of Toxicity Reference Values
3.4.1 Classification
3.4.2 Review of Available Values/Information
3.4.3 Recommendation
3.5 Calculated HILs
3.6 References
4 Endosulfan (total)
4.1 General
4.2 Previous HIL
4.3 Significance of Exposure Pathways
4.3.1 Oral Bioavailability
4.3.2 Dermal absorption
4.3.3 Inhalation of Dust
4.3.4 Plant Uptake
4.3.5 Intakes from Other Sources – Background
4.4 Identification of Toxicity Reference Values
4.4.1 Classification
4.4.2 Review of Available Values/Information
4.4.3 Recommendation
4.5 Calculated HILs
4.6 References
5 Endrin (total)
5.1 General
5.2 Previous HIL
5.3 Significance of Exposure Pathways
5.3.1 Oral Bioavailability
5.3.2 Dermal absorption
5.3.3 Inhalation of Dust
5.3.4 Plant Uptake
5.3.5 Intakes from Other Sources – Background
5.4 Identification of Toxicity Reference Values
5.4.1 Classification
5.4.2 Review of Available Values/Information
5.4.3 Recommendation
5.5 Calculated HILs
5.6 References
6 Heptachlor
6.1 General
6.2 Previous HIL
6.3 Significance of Exposure Pathways
6.3.1 Oral Bioavailability
6.3.2 Dermal absorption
6.3.3 Inhalation of Dust
6.3.4 Plant Uptake
6.3.5 Intakes from Other Sources – Background
6.4 Identification of Toxicity Reference Values
6.4.1 Classification
6.4.2 Review of Available Values/Information
6.4.3 Recommendation
6.5 Calculated HILs
6.6 References
7 Hexachlorobenzene (HCB)
7.1 General
7.2 Previous HIL
7.3 Significance of Exposure Pathways
7.3.1 Oral Bioavailability
7.3.2 Dermal absorption
7.3.3 Inhalation of Dust
7.3.4 Plant Uptake
7.3.5 Intakes from Other Sources – Background
7.4 Identification of Toxicity Reference Values
7.4.1 Classification
7.4.2 Review of Available Values/Information
7.4.3 Recommendation
7.5 Calculated HILs
7.6 References
8 Methoxychlor
8.1 General
8.2 Previous HIL
8.3 Significance of Exposure Pathways
8.3.1 Oral Bioavailability
8.3.2 Dermal absorption
8.3.3 Inhalation of Dust
8.3.4 Plant Uptake
8.3.5 Intakes from Other Sources – Background
8.4 Identification of Toxicity Reference Values
8.4.1 Classification
8.4.2 Review of Available Values/Information
8.4.3 Recommendation
8.5 Calculated HILs
8.6 References
9 Mirex
9.1 General
9.2 Previous HIL
9.3 Significance of Exposure Pathways
9.3.1 Oral Bioavailability
9.3.2 Dermal absorption
9.3.3 Inhalation of Dust
9.3.4 Plant Uptake
9.3.5 Intakes from Other Sources – Background
9.4 Identification of Toxicity Reference Values
9.4.1 Classification
9.4.2 Review of Available Values/Information
9.4.3 Recommendation
9.5 Calculated HILs
9.6 References
10 Toxaphene
10.1 General
10.2 Previous HIL
10.3 Significance of Exposure Pathways
10.3.1 Oral Bioavailability
10.3.2 Dermal absorption
10.3.3 Inhalation of Dust
10.3.4 Plant Uptake
10.3.5 Intakes from Other Sources – Background
10.4 Identification of Toxicity Reference Values
10.4.1 Classification
10.4.2 Review of Available Values/Information
10.4.3 Recommendation
10.5 Calculated HILs
10.6 References
11 Shortened forms
Several comprehensive reviews of DDT, DDE and DDD in the environment and their toxicity to humans are available and should be consulted for more detailed information (ATSDR 2002, 2008; WHO 1979;1989). The following provides a summary of the key aspects of these compounds that are relevant to the derivation of a soil HIL.
Dichlorodiphenyltrichloroethane (DDT), dichlorodiphenyldichloroethylene (DDE) and dichlorodiphenyldichloroethane (DDD) are structurally similar aromatic compounds containing chlorine. Technical DDT was manufactured for broad-spectrum insecticidal usage under a number of trade names including Genitox, Anofex, Detoxan, Neocid, Gesarol, Pentachlorin, Dicophane and Chlorophenothane. Limited manufacture of DDD also occurred under the trade names Rothane, Dilene and TDE (ATSDR 2002).
These compounds are all white crystalline solids with relatively no odour or taste. They are relatively insoluble in water but highly soluble in animal fats and organic solvents (WHO 1979). All three compounds (DDT, DDE, DDD) can exist in different forms (congeners) based on the relative position of chlorines on the two phenyl rings, but the p,p’- congener is the most common in the environment (ATSDR 2002).
DDT was primarily manufactured as an insecticide for the agricultural industry. It was also used to control vector-borne diseases such as malaria and typhus and was used in a domestic setting to protect items from moth damage and to control lice (ATSDR 2002). The widespread usage of DDT reportedly began in 1939. However, it has been progressively banned in many countries since the early 1970s due to its effects on human health and the environment (ATSDR 2002). DDT is still used in some developing regions for the control of disease-bearing insects and it may also be illegally used in the agriculture industry in some countries (WHO 1979). DDT has not been registered for any use in Australia since the 1980s (NHMRC & NRMMC 2011). DDD was manufactured and used to a lesser extent to control insects and the o,p’-isomer was used to medically treat adrenal gland cancer. DDE has no commercial use (ATSDR 2002).
DDT and its metabolites are essentially immobile in soil, becoming strongly absorbed onto the surface layer of soils. Likewise, as a consequence of their extremely low water solubilities, DDT and its metabolites become absorbed onto particulates in water and settle into sediments. Because of its chemical characteristics, DDT can undergo long-range transport through the atmosphere in a process known as ‘global distillation’ where DDT migrates from warmer regions to colder regions through repeated cycles of volatilisation from soil and water surfaces followed by deposition of DDT onto surfaces through dry and wet deposition processes. Due to the persistence of DDT and its metabolites in the environment, potential for bioaccumulation and potential for long-range transport, DDT is listed under the Stockholm Convention on Persistent Organic Pollutants.
The following information primarily relates to DDT which has been adopted as the most appropriate indicator for the assessment of all three compounds due to the similarity of toxic effects and toxicokinetics. A larger database of data is also available for DDT.
The derivation of the previous HIL (HIL A = 200 mg/kg) for DDT+DDE+DDD is presented by Beard (1993) and NEPC (1999). In summary, the HIL was derived on the basis of the following:
Insufficient data is available to adequately define the bioavailability of DDT in the range of contaminated sites that may need to be considered in Australia. On this basis, a default approach of assuming 100% oral bioavailability has been adopted in the derivation of an HIL. It is noted that a site-specific assessment of bioavailability can be undertaken where required.
Dermal absorption of DDT is considered to be very low and has been considered to be negligible in the derivation of the previous HIL. Review of dermal absorption of DDT by MfE (2011) indicated the following: ‘US EPA (2004) recommends a dermal absorption factor of 0.03 (3%), which is based on data from Wester et al. (1990). These authors indicate that only 1.0% of DDT from soil penetrated into human skin over a 24-hour period, and none (<0.1%) of this partitioned into human plasma. Additionally, 3.3% of DDT from soil was absorbed percutaneously following in vivo exposure of rhesus monkeys. Taking the geometric mean of these values yields an average dermal absorption factor of 0.018 (1.8%).’
As few other reviews are available in relation to dermal absorption of DDT, the average value estimated by Wester et al. (1990) as referenced by MfE (2011), has been adopted in the derivation of HILs.
DDT, DDE and DDD are not considered sufficiently volatile to be of significance and inhalation exposures associated with particulates outdoors and indoors are expected to be of less significance than ingestion of soil. While likely to be negligible, potential inhalation exposures associated with dust have been considered in the HIL derived.
As DDT has the potential to bioaccumulate, uptake into fruit and vegetable crops (as well as eggs and poultry where relevant) are likely to be of significance (Beard, 1993). Review by MfE (2011) noted that there are limited studies available for the assessment of plant uptake of DDT, however plant uptake has been considered. It is noted that the few studies available relate to the uptake of DDT in plants when applied in solution (as would be the case as an applied pesticide). DDT, DDE and DDD have a high Koc values (log Koc = 5.195.35, ATSDR 2002) suggesting that these compounds are strongly bound to the soil particulates and immobile in soil (with low solubility in water). For plant uptake to be significant, the chemicals must be able to partition to soil water. In addition there is evidence that DDT, as well as other chemicals, undergoes an ageing process in soil whereby the DDT is sequestered in the soil so decreasing its bioavailability to microorganisms, extractability with solvents, and toxicity to some organisms (ATSDR 2002).
ATSDR (2002) reviewed available studies associated with potential uptake of DDT that is sorbed to soil. The studies show that the potential for uptake was low and there was little or no evidence of translocation. Some uptake was noted where the DDT source was fresh and some volatilisation had occurred resulting in uptake, though this process is not consider relevant for most DDT-contaminated sites as these compounds are no longer used in Australia.
On the basis of the above, the potential for plant uptake of DDT, DDE and/or DDD bound to the soil is considered to be negligible.
It is noted, however, that should these compounds be present in other media such as groundwater (used for irrigation) or in solution then the potential for uptake into fruit and vegetable crops is likely to be of significance. These issues should be assessed on a site-specific basis
For the general population, background intakes would be expected to be primarily associated with residues in food, which appear to be slowly disappearing from the food chain (Beard 1993). Background intakes considered in the previous HIL were estimated to be 0.546 mg/kg/day for infants, predominantly derived from dietary sources.
More recent information from Food Standards Australia New Zealand in the 20th Australian Total Diet Survey (FSANZ 2003) indicates that dietary exposures for all age groups was less than 0.2% of the adopted ADI (0.002 mg/kg/day). DDT was not detected in the 23rd Australian Total Diet Survey (FSANZ 2011). On this basis, background intakes can be considered negligible (0%). This evaluation is consistent with that presented by RIVM (2001).
The International Agency for Research on Cancer (IARC 1991) has classified DDT and associated compounds as 2B—possible human carcinogens.
The US EPA has classified DDT as B2—probable human carcinogen.
While DDT has some carcinogenic potential, the mode of action is important in determining the most appropriate approach to the identification of quantitative toxicity values. No discussion is presented in the profile regarding mode of action and potential for genotoxicity. Review of available information (ATSDR 2002; WHO (2011); RIVM 2001; IARC 1991) suggests that while some conflicting data is available with regard to some genetic end points, DDT and derivatives are not genotoxic (or it is equivocal) and therefore it is not appropriate to consider a non-threshold (linear) doseresponse approach. Hence, it is not appropriate to consider the use of the slope factor and unit risk values available from US EPA.
On the basis of the available information, it is considered appropriate that a threshold doseresponse approach be adopted for DDT. The following are available from Level 1 Australian and International sources:
Source | Value | Basis/Comments |
Australian | ||
ADWG (NHMRC 2011) | TDI = 0.0025 mg/kg/day | The NHMRC derived a guideline of 0.009 mg/L based on a TDI of 0.0025 mg/kg/day. The TDI is derived on the basis of a NOEL of 0.25 mg/kg/day from a 25-year study in humans, and an uncertainty factor of 100 (includes 10 for intraspecies variation and 10 for the uncertainty arising from the lack of detail in the epidemiological study used). |
OCS (2012) | TDI = 0.002 mg/kg/day | TDI was set in 2003 (same as previous ADI), based on a NOEL of 0.25 mg/kg/day from studies in humans and experimental animals, and an uncertainty factor of 100. |
International | ||
WHO (2011) | PTDI = 0.01 mg/kg/day | Provisional TDI referenced inWHO(2011) was established by JMPR in 2000 (as published by JMPR 2001) based on a NOAEL of 1 mg/kg/day for developmental toxicity in rats, and a safety factor of 100. |
RIVM (2001) | TDI = 0.0005 mg/kg/day
| TDI based on a NOAEL of 0.05 mg/kg/day associated with hepatotoxic effects in a 15 to 27-week study in female rats, and a 100-fold uncertainty factor. |
ATSDR (2002) | No chronic value derived | Not derived as most sensitive non-cancer effects were observed at doses higher than doses for most sensitive acute and intermediate duration effects. The acute and intermediate MRL derived for DDT was 0.0005 mg/kg/day. |
US EPA (IRIS 2012) | RfD = 0.0005 mg/kg/day
| The US EPA review (last updated in 1987) derived an oral RfD on the basis of a NOEL of 0.05 mg/kg/day associated with liver effects in a rat study (1950 study), and uncertainty factor of 100. The US EPA has also derived an oral slope factor and inhalation unit risk, however these are not considered appropriate for the assessment of a non-genotoxic carcinogen. |
There is a wide range of threshold values available for oral intakes of DDT. It is recommended that the oral value available from OCS (2012) ,which is consistent with the value in the ADWG (NHMRC 2011), be adopted for the derivation of an Australian HIL. The US EPA evaluation, while providing a more conservative TRV, has not been considered as it is significantly dated, using a key study from 1950. Reviews conducted by WHO and NHMRC are more current and have considered more recent studies.
No dermal or inhalation-specific studies or data are available. For the presence of DDT (DDE and DDD) in soil, it is considered appropriate to consider use of the available ADI for all pathways of exposures.
On the basis of the discussion above, the following toxicity reference values (TRVs) have been adopted for DDT, DDE and DDD (in total) in the derivation of HILs:

On the basis of the above, the following HILs have been derived for DDT+DDE+DDD (refer to Appendix B for equations used to calculate the HILs and Appendix C for calculations):
HIL Scenario | HIL (mg/kg) | Percentage Contribution from Exposure Pathways | |||
Ingestion of Soil/Dust | Ingestion of Home-grown Produce | Dermal Absorption of Soil/Dust | Inhalation (dust) | ||
Residential A | 240 | 80 | -- | 20 | <1 |
Residential B | 600 | 51 | -- | 49 | <1 |
Recreational C | 400 | 67 | -- | 33 | <1 |
Commercial D | 3600 | 42 | -- | 58 | <1 |
-- Pathway not included in derivation of HIL
ATSDR 2002, Toxicological Profile for DDT, DDE and DDD, US Department of Health and Human Services, ATSDR, available from: http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=81&tid=20.
ATSDR 2008, Addendum to the DDT/DDD/DDE Toxicological Profile. US Department of Health and Human Services, ATSDR, available from http://www.atsdr.cdc.gov/toxprofiles/ddt_addendum.pdf.
Beard 1993, ‘The Evaluation of DDT Contaminated Soil Associated with Cattle Tick Dip Sites’, presented in the proceedings of the Second National Workshop on the Health Risk Assessment and Management of Contaminated Sites, Contaminated Sites Monograph Series, No. 2.
FSANZ 2003, The 20th Australian Total Diet Survey, A total diet survey of pesticide residues and contaminants, Food Standards Australia and New Zealand.
FSANZ 2011, The 23rd Australian Total Diet Study, Food Standards Australia and New Zealand.
IARC 1991, Summaries & Evaluations. DDT and Associated Compounds, vol. 53, (1991), p.179, International Agency for Research on Cancer.
JMPR1985, Pesticide residues in food — 1984 evaluations, Food and Agriculture Organization of the United Nations, Rome, Joint FAO/WHO Meeting on Pesticide Residues (FAO Plant Production and Protection Paper 67).
JMPR 2001, Pesticide residues in food 2000. Evaluations 2000. Part II Toxicology, World Health Organization, Geneva, Joint FAO/WHO Meeting on Pesticide Residues (WHO/PCS/01.3).
MfE 2011, Toxicological intake values for priority contaminants in soil, New Zealand Ministry for the Environment, Wellington, New Zealand.
NEPC 1999, Schedule B (7a), Guideline on Health-Based Investigation Levels, National Environment Protection (Assessment of Site Contamination) Measure, National Environment Protection Council, Australia.
NHMRC & NRMMC 2011, National water quality management strategy, Australian drinking water guidelines, National Health and Medical Research Council and National Resource Management Ministerial Council, Australia.
OCS 2012, ADI List, Acceptable Daily Intakes for Agricultural and Veterinary Chemicals, Current to 31 March 2012, Australian Government, Department of Health and Ageing, Office of Chemical Safety (OCS), available from: http://www.health.gov.au/internet/main/publishing.nsf/content/E8F4D2F95D616584CA2573D700770C2A/$File/ADI-apr12.pdf.
RIVM 2001, Re-evaluation of human-toxicological Maximum Permissible Risk levels. National Institute of Public Health and the Environment, Bilthoven, Netherlands, available from: http://www.rivm.nl/bibliotheek/rapporten/711701025.html.
US EPA (IRIS 2012), Data and information available from the Integrated Risk Information System, an online database, available from http://www.epa.gov/iris/.
Weste,r RC, Maibach, HI, Bucks, DAW, Sedik, L, Melendres, J, Liao, C & DiZio, D 1990, ‘Percutaneous absorption of [14C]DDT and [14C]benzo[a]pyrene from soil’, Fundamental and Applied Toxicology, vol. 15, pp. 510–516.
WHO 1979, DDT and its Derivatives, Environmental Health Criteria 9, available from: http://www.inchem.org/documents/ehc/ehc/ehc009.htm.
WHO 1989, DDT and its Derivatives – Environmental Aspects, Environmental Health Criteria 83. available from http://www.inchem.org/documents/ehc/ehc/ehc83.htm.
WHO 2011, Guidelines for drinking-water quality, 4th edn, World Health Organization, Geneva, available from http://www.who.int/water_sanitation_health/dwq/chemicals/en/index.html.
Several comprehensive reviews of aldrin and dieldrin in the environment and their toxicity to humans are available and should be consulted for more detailed information (ATSDR 2002; WHO 1989). The following provides a summary of the key aspects of these compounds that are relevant to the derivation of a soil HIL.
Aldrin and dieldrin are the common names of two structurally similar compounds that were historically used as insecticides. These two chemicals are discussed together because aldrin is readily converted to dieldrin once it enters either the environment or the body, and both compounds reportedly have similar health effects. Aldrin predominantly contained the compound hexachlorohexahydrodimethanonaphthalene (HHDN) and was also produced under the following trade names; Aldrec, Aldrex, Drinox, Octalene, Seedrin, and Compound 118 (ATSDR 2002). Technical-grade aldrin contained not less than 85% aldrin with common impurities including isodrin, hexachlorobutadiene, chlordane, octachlorocyclopentene and toluene (ATSDR 2002).
Dieldrin was manufactured by the epoxidation of aldrin. Technical grade dieldrin, which was also produced under the trade names Alvit, Dieldrix, Octalox and Red Shields, contained no less that 85% by weight hexachloroepoxyoctahydrodimethanonaphthalene (HEOD). Pure HHDN and HEOD are structurally similar, stable white powders or crystals with a mild chemical odour. Commercial grade aldrin and dieldrin are tan coloured powders. Aldrin and dieldrin have low vapour pressure and are relatively insoluble in water (ATSDR 2002).
Aldrin and dieldrin, which do not occur naturally in the environment, were synthesised for commercial use as contact insecticides. Both chemicals were widely used against soil-dwelling pests in agriculture, particularly in the corn and citrus industries (ATSDR 2002). They were also used for the protection of wood structures or electrical and telecommunication cables against termites and woodborers.
They were used in Australia and across the world from the 1950s until their commercial distribution was restricted in the 1970s. By the end of 1980, there was a significant reduction on the number of aldrin and dieldrin products formally approved. All uses of these products were deregistered by 1985.
The derivation of the previous HIL (HIL A = 10 mg/kg) for aldrin and dieldrin is presented by Di Marco (1993) and NEPC (1999). In summary, the HIL was derived on the basis of the following:
Insufficient data is available to adequately define the bioavailability of aldrin and dieldrin in the range of contaminated sites that may need to be considered in Australia. On this basis, a default approach of assuming 100% oral bioavailability has been adopted in the derivation of an HIL. It is noted that a site-specific assessment of bioavailability can be undertaken where required.
A proposed range for dermal absorption of pesticides from soil was 1%10% (Ryan et al. 1987). The reported absorption of topically applied pesticides and herbicides in acetone to in vitro human skin was reported to be within this range for lindane, aldrin, dieldrin, malathion, parathion, and 2,4-D in Feldmann & Maibach, (1974). WHO (1989) noted that aldrin and dieldrin are readily absorbed by oral, inhalation and dermal routes. Absorption through the intact skin was about 78% of the applied dose in a human volunteer study. On this basis, adopting the default of 0.1 (10%) recommended by US EPA (1995) for pesticides is considered reasonable.
Aldrin and dieldrin are not considered sufficiently volatile to be of significance and inhalation exposures associated with particulates outdoors and indoors are expected to be of less significance than ingestion of soil. While likely to be negligible, potential inhalation exposures associated with dust have been considered in the derivation of soil HILs.
Aldrin and dieldrin have the potential to bioconcentrate in terrestrial ecosystems. However, the available studies show mixed results with respect to plant uptake. Some studies show potential uptake (more significantly into roots) while others have shown no plant uptake (from these compounds bound to soil) (ATSDR 2002). Both aldrin and dieldrin have high Koc values (log Koc = 6.677.67, ATSDR 2002), suggesting that these compounds are largely bound to soil particulates and immobile in soil. For plant uptake to be significant, the chemicals must be able to partition to soil water. With respect to aldrin and dieldrin bound to the soil, this is considered to be insignificant. Hence, the potential for plant uptake of aldrin and dieldrin from soil contamination is considered negligible.
It is noted, however that should these compounds be present in other media such as groundwater (used for irrigation) or solution, then the potential for uptake into fruit and vegetable crops is likely to be of significance. In addition, the mobility of these compounds in the soil environment can be enhanced by the presence of organic solvents. These organic solvents have the ability to increase the water solubility of non-polar compounds, which in turn increases their mobility in soil. The organic solvents in a sense act as a transport medium for chemicals that would normally bind strongly to soil (ATSDR 2002). These issues should be assessed on a site-specific basis.
For the general population where aldrin and dieldrin are no longer used, background intakes would be expected to be primarily associated with residues in food. Food Standards Australia and New Zealand has not detected aldrin or dieldrin in any sample in the 19th or 20th food surveys (FSANZ 2003). Dieldrin was reported (at 1.283.23% of the ADI adopted) in the earlier 18th survey and again in the most recent survey (FSANZ 2011) with the highest intake estimated to be 0.021 µg/kg/day for children aged 25 years, which comprises 20% of the adopted oral TRV. Other than the most recent food survey, intakes of aldrin and dieldrin are negligible, however the higher level of intake estimated for young children more recently suggests intakes are not negligible. For the purpose of establishing a soil HIL, an intake of 10% (assumed to represent a longer-term average of intakes from the available food surveys) of the TRV from other sources has been assumed.
The International Agency for Research on Cancer (IARC) has classified aldrin and dieldrin as Group 3—not classifiable as to carcinogenicity to humans. It is noted that US EPA has classified both aldrin and dieldrin as Class B2—probable human carcinogens.
There are mixed reviews of carcinogenicity with respect to aldrin and dieldrin. Based on a review by RIVM (2001) it is noted that epidemiological data remains inadequate, though some studies have shown hepatocellular carcinomas in mice, while other studies have not shown carcinogenic effects. Further evaluation of carcinogenicity by Stevenson et al. (1999) within the framework of the US EPA Proposed Guidelines for Carcinogen Risk Assessment considered that dieldrin-induced liver tumours occur through a non-genotoxic mode of action. The review also considers that a more appropriate cancer descriptor for aldrin/dieldrin is ‘not likely to be carcinogenic to humans’.
On the basis of the available information, it is considered appropriate that a threshold doseresponse approach be adopted for aldrin and dieldrin. The following are available from Level 1 Australian and International sources:
Source | Value | Basis/Comments |
Australian | ||
ADWG (NHMRC & NRMMC 2011) | ADI = 0.0001 mg/kg/day | The ADI available in the ADWG (NHMRC & NRMMC 2011) is noted to be derived from JMPR evaluation (noted to be 1977). |
OCS (2012) | TDI = 0.0001 mg/kg/day | This value (set in 2003) is based on the JMPR evaluation from 1994 (refer to comment below). The TDI is noted to be retained for comparison against dietary intakes only as these compounds are no longer used in agricultural practice. The ADI listed is also adopted by FSANZ. |
International | ||
WHO (2011) | PTDI = 0.0001 mg/kg/day | The ADI/PTDI has been considered in the derivation of WHO (2011) based on the JMPR evaluation where the provisional TDI is based on a NOAEL of 1 mg/kg/day in dogs and 0.5 mg/kg/day in rats (dietary studies, Fitzhugh et al. 1964; Fitzhugh & Nelson 1963), which is equivalent to 0.025 mg/kg/day in both species, and application of 250-fold uncertainty factor (10 for interspecies variation, 10 for intraspecies variation and 2.5 for concern about carcinogenicity observed in mice). It is noted that the WHO DWG evaluation has not changed since 1970. |
RIVM (2001) | Adopted JMPR evaluation as noted above from WHO DWG | |
ATSDR (2002) | MRL = 0.00003 mg/kg/day for aldrin MRL = 0.00005 mg/kg/day for dieldrin | Chronic oral MRLs for aldrin and dieldrin based on a LOAEL of 0.025 mg/kg/day associated with liver effects in rats and application of 100-fold uncertainty factor. Values adopted are consistent with those available from the US EPA (IRIS 2012). |
US EPA (IRIS 2012) | RfD = 0.00003 mg/kg/day for aldrin RfD = 0.00005 mg/kg/day for dieldrin
| RfD for aldrin based on a LOAEL of 0.025 mg/kg/day associated with liver toxicity in a chronic rat study (Fitzhugh et al. 1964) and application of a 1000-fold uncertainty factor. The evaluation was last reviewed in 1988. RfD for dieldrin based on a NOAEL of 0.005 associated with liver lesions in a 2-year rat study and a 100-fold uncertainty factor. The review was last updated in 1990. In addition the US EPA has also derived non-threshold values for aldrin and dieldrin. It is not considered appropriate to quantify aldrin and dieldrin toxicity on the basis of a non-threshold approach. |
All the key studies considered in the above reviews are dated (in the 1960s) and no new studies are available that suggest the evaluations provided and adopted in the Australian and WHO drinking water guidelines are not current. Hence the oral value adopted by NHMRC & NRMMC (2011) and WHO (2011) provide a suitable basis for the derivation of a soil HIL. No dermal or inhalation-specific studies or data are available. For the presence of aldrin and dieldrin in soil, it is considered appropriate to consider use of the available TRV for all pathways of exposures.
On the basis of the discussion above, the following toxicity reference values (TRVs) have been adopted for aldrin and dieldrin in the derivation of HILs:

On the basis of the above, the following HILs have been derived for aldrin and dieldrin (refer to Appendix B for equations used to calculate the HILs and Appendix C for calculations):
HIL Scenario | HIL (mg/kg) | Percentage Contribution from Exposure Pathways | |||
Ingestion of Soil/Dust | Ingestion of Home-grown Produce | Dermal Absorption of Soil/Dust | Inhalation (dust) | ||
Residential A | 6 | 43 | -- | 57 | <1 |
Residential B | 10 | 16 | -- | 84 | <1 |
Recreational C | 10 | 27 | -- | 73 | <1 |
Commercial D | 45 | 12 | -- | 88 | <1 |
-- Pathway not included in derivation of HIL
ATSDR 2002, Toxicological Profile for Aldrin/Dieldrin US Department of Health and Human Services, ATSDR, September 2002, available from http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=317&tid=56.
Di Marco, P 1993, ‘The Assessment and Management of Organochlorine Termiticides’, presented in the proceedings of the Second National Workshop on the Health Risk Assessment and Management of Contaminated Sites, Contaminated Sites Monograph Series, No. 2.
Feldmann, RJ & Maibach, HI 1974, ‘Percutaneous penetration of some pesticides and herbicides in man’, Toxicol. Appl. Pharmacol, vol. 28, pp. 126132.
Fitzhugh, OG & Nelson, AA 1963, Unpublished data from the US Food and Drug Administration, as cited in JMPR, 1967.
Fitzhugh, OG, Nelson, AA & Quaife, ML 1964, ‘Chronic oral toxicity of aldrin and dieldrin in rats and dogs’, Food Cosmet Toxicol, vol. 2, pp. 551562.
FSANZ 2003, The 20th Australian Total Diet Survey, A total diet survey of pesticide residues and contaminants, Food Standards Australia and New Zealand.
FSANZ 2011, The 23rd Australian Total Diet Study, Food Standards Australia and New Zealand.
IARC 1991, Summaries & Evaluations. DDT and Associated Compounds, vol. 53, (1991), p.179, International Agency for Research on Cancer.
NEPC 1999, Schedule B (7a), Guideline on Health-Based Investigation Levels, National Environment Protection (Assessment of Site Contamination) Measure, National Environment Protection Council, Australia.
NHMRC 2011, National water quality management strategy. Australian drinking water guidelines, National Health and Medical Research Council, Australia.
OCS 2012, ADI List, Acceptable Daily Intakes for Agricultural and Veterinary Chemicals, current to 31 March 2012, Australian Government, Department of Health and Ageing, Office of Chemical Safety (OCS), available from: http://www.health.gov.au/internet/main/publishing.nsf/content/E8F4D2F95D616584CA2573D700770C2A/$File/ADI-apr12.pdf.
RIVM 2001. Re-evaluation of human-toxicological Maximum Permissible Risk levels, National Institute of Public Health and the Environment, Bilthoven, Netherlands,, available from: http://www.rivm.nl/bibliotheek/rapporten/711701025.html.
Ryan, EA, Hawkins, ET et al. 1987, ‘Assessing Risk from Dermal Exposure at Hazardous Waste Sites’, in Bennett, G & Bennett, J, (eds), Superfund '87: Proceedings of the Eighth National Conference, November 16-18, pp. 166168, The Hazardous Materials Control Research Institute, Washington, DC.
Stevenson, DE, Walborg, .F, North, DW, Sielken Jr, RL, Ross, CE, Wright, AS, Xu, Y, Kamendulis, LM & Klaunig, JE 1999, ‘Monograph: Reassessment of human cancer risk of aldrin/dieldrin’, Toxicology Letter, vol. 109, Issues 3, October 1999, pp. 123186.
US EPA (IRIS 2012), Data and information available from the Integrated Risk Information System, an online database, available from http://www.epa.gov/iris/.
US EPA 1995, Technical Guidance Manual, Assessing Dermal Exposure from Soil, US EPA Region 3, available from: http://www.epa.gov/reg3hwmd/risk/human/info/solabsg2.htm.
WHO 1989, Aldrin and dieldrin. Environmental Health Criteria 91, International Programme on Chemical Safety, World Health Organization Geneva.
WHO 2011, Guidelines for drinking-water quality, 4th edn, World Health Organization, Geneva, available from http://www.who.int/water_sanitation_health/dwq/chemicals/en/index.html.
Several comprehensive reviews of chlordane in the environment and its toxicity to humans are available and should be consulted for more detailed information not presented in this summary (ATSDR 1994). The following provides a summary of the key aspects of chlordane that are relevant to the derivation of a soil HIL.
Chlordane is a manufactured chemical that does not occur naturally in the environment. It is a thick liquid, and the colour ranges from colourless to amber depending on its purity. Chlordane does not dissolve in water but can be produced as an emulsion enabling it to be sprayed (ATSDR 1994). Sixty to eighty-five percent of chlordane consists of the stereo-isomers cis- and trans-chlordane, with the remainder comprising a number of impurities (ATSDR 1994).
Chlordane is a manufactured chemical that was used as a broad-spectrum insecticide in the United States between 1948 and 1988 (ATSDR 1994). In Australia, it was used to protect wooden structures against termites until June 1995 (NHMRC 2011). Some of its trade names are Octachlor and Velsicol 1068.
The derivation of the previous HIL (HIL A = 50 mg/kg) for chlordane is presented by Di Marco (1993) and NEPC (1999). In summary, the HIL was derived on the basis of the following:
Insufficient data is available to adequately define the bioavailability of chlordane in the range of contaminated sites that may need to be considered in Australia. On this basis, a default approach of assuming 100% oral bioavailability has been adopted in the derivation of an HIL. It is noted that a site-specific assessment of bioavailability can be undertaken where required.
US EPA (2004) has identified a dermal absorption fraction of 0.04 (4%), based on a study by Wester et al. (1992) for chlordane in soil. No additional data is available to suggest more significant dermal absorption values are relevant for chlordane.
Chlordane is not considered sufficiently volatile to be of significance and inhalation exposures associated with particulates outdoors and indoors are expected to be of less significance than ingestion of soil. While likely to be negligible, potential inhalation exposures associated with dust have been considered in the HIL derived.
Chlordane has the potential to bioconcentrate in terrestrial ecosystems. However, there are few studies available on the potential for plant uptake. Chlordane has a high Koc value (log Koc = 3.496.3, ATSDR 1994) suggesting that the compound is largely bound to soil particulates and immobile in soil. In addition, chlordane has a low solubility in water. For plant uptake to be significant, the chemicals must be able to partition to soil water. Information available from EFSA (2007) notes that chlordane is considered a non-systemic (not taken up by the plant) insecticide. Hence, with respect to chlordane bound to the soil, this is considered to be insignificant and negligible.
The review presented by Di Marco (1993) in the derivation of the previous HIL included a review of intakes (using available Australian data) that may be derived from water, air (including homes where termiticide treatment had occurred), soil and food. It is noted that use of chlordane was phased out in all states/territories except the Northern Territory in 1995. In 1997, chlordane was allowed to be used in the Northern Territory until stocks of the product were exhausted. Chlordane is now banned in Australia and hence background intakes estimated by Di Marco (1993) associated with product use are no longer relevant.
Background intakes of chlordane (where the product is not used) range from 0.1 ng/kg/day for adults to 0.46 ng/kg/day for children (where food intakes were most significant). Food Standards Australia and New Zealand has not detected chlordane in any sample in the 19th, 20th or 23rd food surveys (FSANZ 2003; FSANZ 2011). Hence, background intakes would be expected to be negligible. Assuming a negligible background intake is considered appropriate, based on current information.
The International Agency for Research on Cancer (IARC 2001) has classified chlordane as Group 2B—possibly carcinogenic to humans.
It is also noted that US EPA has classified chlordane as B2—probable human carcinogen (last reviewed in 1998).
As chlordane has been banned from use in a number of countries, there are few recent studies/reviews available. Review of chlordane by the European Food Safety Committee (EFSA 2007) provided a review of long-term toxicity studies, carcinogenicity and genotoxicity for chlordane. Long-term oral studies with the nervous system and liver were shown to be the most significant target organs. Data on genotoxicity is limited and conflicting, however overall chlordane was not mutagenic in vivo and not or only weakly mutagenic in a few tests in vitro. On the basis of the weight of evidence, chlordane is not considered to be genotoxic. Chlordane causes liver tumours in mice via a non-genotoxic mechanism and is classified by IARC as possibly carcinogenic to humans.
On the basis of the available information, it is considered appropriate that a threshold doseresponse approach be adopted for chlordane. The following are available from Level 1 Australian and International sources:
Source | Value | Basis/Comments |
Australian | ||
ADWG (NHMRC 2011) | ADI = 0.00045 mg/kg/day | Current ADWG (NHMRC 2011) of 0.001 mg/L based on 10% intake from drinking water, a NOEL of 0.045 mg/kg/day based on a long-term (30 week) dietary study in rats, and a 100-fold uncertainty factor. This is consistent with the PTDI used in the current WHO DWG, as well as OCS (2012). |
OCS (2012) | TDI = 0.0005 mg/kg/day | TDI was set in 2003, no study referenced. This value is noted to be based on the JMPR evaluation from 1994. The TDI is noted to be retained for comparison against dietary intakes only as these compounds are no longer used in agricultural practice. The TDI listed is also adopted by FSANZ. |
International | ||
WHO (2011) | PTDI = 0.0005 mg/kg/day | Provisional TDI based on a NOAEL of 0.05 mg/kg/day for increased liver weights, serum bilirubin levels and hepatocellular swelling from a long term study in rats (same study as considered in the ADWG), and a 100-fold uncertainty factor. |
ATSDR (1994) | Oral MRL = 0.0006 mg/kg/day Inhalation MRL = 0.00002 mg/m3 | Chronic oral MRL based on liver hypertrophy in a 30-month rat study. Chronic inhalation MRL based on hepatic effects in a 90-day subchronic rat study. The study used to derive the inhalation MRL is the same as that used by the US EPA in the derivation of the RfC. The application of uncertainty factors differs between the organisations. |
US EPA (IRIS 2012) | RfD = 0.0005 mg/kg/day RfC = 0.0007 mg/m3
| Oral RfD based on a NOAEL of 0.15 mg/kg/day associated with hepatic necrosis in a 104-week mouse study, and 300-fold uncertainty factor. RfC based on hepatic effects in a subchronic rat inhalation study. The evaluation was last reviewed in 1998. In addition, US EPA has also derived an oral cancer slope factor and an inhalation unit risk (based on the oral evaluation). It is not considered appropriate to quantify chlordane toxicity on the basis of a non-threshold approach. |
Based on the available data above, there is general agreement on the consideration of an oral TRV of 0.0005 mg/kg/day. Limited inhalation data is available, with the US EPA RfC essentially equivalent to the oral TRV; hence it is recommended that all intakes associated with contaminated soil be assessed on the basis of the oral TRV.
On the basis of the discussion above, the following toxicity reference values (TRVs) have been adopted for chlordane in the derivation of HILs:
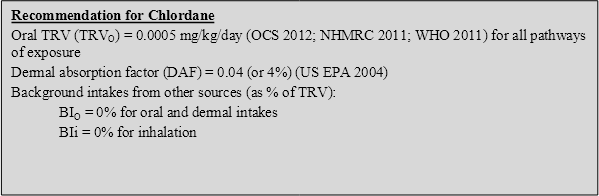
On the basis of the above, the following HILs have been derived for chlordane (refer to Appendix B for equations used to calculate the HILs and Appendix C for calculations):
HIL Scenario | HIL (mg/kg) | Percentage Contribution from Exposure Pathways | |||
Ingestion of Soil/Dust | Ingestion of Home-grown Produce | Dermal Absorption of Soil/Dust | Inhalation (dust) | ||
Residential A | 50 | 65 | -- | 35 | <1 |
Residential B | 90 | 32 | -- | 68 | <1 |
Recreational C | 70 | 48 | -- | 52 | <1 |
Commercial D | 530 | 25 | -- | 75 | <1 |
-- Pathway not included in derivation of HIL
ATSDR 1994, Toxicological Profile for Chlordane, US Department of Health and Human Services, ATSDR, available from http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=355&tid=62.
Di Marco, P 1993 ‘The Assessment and Management of Organochlorine Termiticides’, presented in the proceedings of the Second National Workshop on the Health Risk Assessment and Management of Contaminated Sites, Contaminated Sites Monograph Series, No. 2, 1993.
EFSA 2007, ‘Chlordane as undesirable substance in animal feed, Scientific Panel on contaminants in the Food Chain’, The EFSA Journal, vol. 582, pp. 152, European Food Safety Authority, adopted 7 November 2007.
FSANZ 2003, The 20th Australian Total Diet Survey, A total diet survey of pesticide residues and contaminants, Food Standards Australia and New Zealand.
FSANZ 2011, The 23rd Australian Total Diet Study, Food Standards Australia and New Zealand.
IARC 2001, Summaries & Evaluations, Chlordane and Heptachlor, Vol. 79, p.411, International Agency for Research on Cancer.
NEPC 1999, Schedule B (7a), Guideline on Health-Based Investigation Levels, National Environment Protection (Assessment of Site Contamination) Measure, National Environment Protection Council, Australia.
NHMRC 2011, National water quality management strategy, Australian drinking water guidelines, National Health and Medical Research Council, Australia.
OCS 2012, ADI List, Acceptable Daily Intakes for Agricultural and Veterinary Chemicals, current to 31 March 2012, Australian Government, Department of Health and Ageing, Office of Chemical Safety (OCS), available from: http://www.health.gov.au/internet/main/publishing.nsf/content/E8F4D2F95D616584CA2573D700770C2A/$File/ADI-apr12.pdf.
US EPA 2004, Risk Assessment Guidance for Superfund, Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment), Final, EPA/540/R-99/005, OSWER 9285.7-02EP.
US EPA (IRIS 2012), data and information available from the Integrated Risk Information System, an online database, available from http://www.epa.gov/iris/.
Wester, RC, Maibach, HI, Sedik, L, Melendres, J, Laio, CL, & DeZio, S 1992, ‘Percutaneous Absorption of [14C]Chlordane from Soil’, J. Toxicol. Environ. Health, vol. 35, pp. 269277.
WHO 2011, Guidelines for drinking-water quality, 4th edn, World Health Organization, Geneva, available from http://www.who.int/water_sanitation_health/dwq/chemicals/en/index.html.
Several comprehensive reviews of endosulfan in the environment and its toxicity to humans are available and should be consulted for more detailed information (ATSDR 2000; APVMA 2005; Marshall & Rutherford 2003; WHO 1984). The following provides a summary of the key aspects of endosulfan that are relevant to the derivation of a soil HIL.
Endosulfan is the common name for an organochlorine pesticide which predominantly contains the compound 6,7,8,9,10,10-hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3-benzodioxathiepine-3-oxide. The manufacture of endosulfan yields two stereo-isomers denoted as α-endosulfan and β-endosulfan in a ratio of 7:3. Technical grade endosulfan generally comprises 94% total endosulfan (α-endosulfan and β-endosulfan) with the remainder comprising impurities and degradation products such as endosulfan ether, endosulfan alcohol and endosulfan sulfate (ATSDR 2000). Endosulfan insecticides are produced under the following trade names; Thiodan; Thionex; Thionate Malix; HOE 2671; FMC 5462; Cyclodan; Thifor; Beosit; Chlorthiepin and Endocide (ATSDR 2000).
Endosulfan is a cream to brown-coloured crystalline solid or waxy solid with a distinct turpentine-like odour. It has a low solubility in water, a low vapour pressure and does not burn (ATSDR 2000). The β-isomer is more chemically stable than the α-isomer, which slowly transforms to the β-isomer in the environment (ATSDR 2000).
Endosulfan is manufactured and used as a broad-spectrum insecticide to control insects on horticultural and agricultural products such as grains, tea, fruits, vegetables, tobacco and cotton. It is manufactured worldwide for commercial agricultural use and domestic (home gardening) use. Endosulfan is also used as a wood preservative (ATSDR 2000). Endosulfan was registered for commercial use in Australia in the 1970s until its deregistration in October 2010 with a two-year phase-out until October 2012. Prior to the late 1990s, when restrictions on its use were introduced, approximately 900 tonnes of technical grade endosulfan was imported annually into Australia. Its use significantly decreased in the years leading up to its deregistration. It was primarily used in cotton production (70%), followed by vegetables (20%) and other crops and horticultural products (10%) (APVMA 2005). Both isomers of endosulfan and endosulfan sulphate were added to the Stockholm Convention in April 2011.
No previous HIL is available (NEPC 1999), though it is noted that review of endosulfan by Marshall & Rutherford (2003) suggested a soil guideline value of 160 mg/kg may be derived (assuming 20% of ADI is derived from soil, 100% bioavailability and soil ingestion is the most significant pathway of exposure).
Insufficient data is available to adequately define the bioavailability of endosulfan in the range of contaminated sites that may need to be considered in Australia. On this basis, a default approach of assuming 100% oral bioavailability has been adopted in the derivation of an HIL. It is noted that a site-specific assessment of bioavailability can be undertaken where required.
Insufficient data is available on the dermal absorption of endosulfan from soil. Hence the default values of 0.1 (10%) suggested by US EPA (1995) for pesticides has been adopted in the derivation of HILs.
Endosulfan is not considered sufficiently volatile to be of significance and inhalation exposures associated with particulates outdoors and indoors are expected to be of less significance than ingestion of soil. While likely to be negligible, potential inhalation exposures associated with dust have been considered in the HIL derived.
The few studies that are available with respect to the potential for plant uptake of endosulfan relate to the application of endosulfan in solution, rather than uptake from soil. Endosulfan has a high Koc value (log Koc = 3.5) and low solubility in water (ATSDR 2000), suggesting that the compound is largely bound to soil particulates and immobile in soil. For plant uptake to be significant, the chemicals must be able to partition to soil water. With respect to endosulfan bound to the soil, the potential for partitioning to soil water is considered to be low and hence plant uptake is considered to be negligible.
Background intakes have been assessed by Marshall & Rutherford (2003) on the basis of available Australian data. For a 2-year-old child, background intakes (from air, food and water) were estimated to contribute 7% of the ADI adopted (0.006 mg/kg/day). However, it has been noted that this evaluation was based on limited data and a default approach of considering 80% background intakes was adopted.
Background exposure by the general public is expected to be dominated by food residue intakes in areas away from where endosulfan products are being applied. Food Standards Australia and New Zealand has reported that intakes of endosulfan by all age groups was less than or equal to 2% of the adopted ADI in the 23rd Australian Total Diet Study (FSANZ 2011). The National Estimated Daily Intake of endosulfan was reviewed by APVMA (2005) and estimated to be equivalent to 27% of the recommended oral TRV (0.006 mg/kg/day), which is more conservative that the current dietary survey indicates. On this basis a background intake of 30% is considered appropriate for deriving a soil HIL for endosulfan.
The International Agency for Research on Cancer (IARC) and US EPA have not classified endosulfan with respect to human carcinogenicity.
Limited data is available to assess carcinogenicity of endosulfan. Evaluation of the WHO DWG (WHO 2011) referenced JMPR (WHO 1998), who concluded that endosulfan is not genotoxic and no carcinogenic effects have been noted in long-term studies in rats and mice. This is also noted, in the NRA (1998) review. Review by APVMA (2005) has reassessed the potential for endosulfan to be an endocrine disruptor. The review concluded that the endocrine-disrupting potential of the compound was not a significant risk to public health under the existing management controls and health standards.
On the basis of the available information, it is considered appropriate that a threshold doseresponse approach be adopted for endosulfan and that no additional consideration is required to address endocrine-disrupting effects. The following are available from Level 1 Australian and International sources:
Source | Value | Basis/Comments |
Australian | ||
ADWG (NHMRC, 2011) | TDI = 0.006 mg/kg/day | The NHMRC derived a guideline of 0.02 mg/L from a TDI of 0.006 mg/kg/day that is based on a NOEL of 0.57 mg/kg/day from a 1-year dietary study in dogs, and an uncertainty factor of 100. |
OCS (2012) and FSANZ (2011) | ADI = 0.006 mg/kg/day | ADI was set in May 1997 and based on a NOEL of 0.6 mg/kg/day from a 78-week dietary study in mice, 13-week dietary study in rats, 1-year dietary study in dogs and a developmental study in rats. The ADI is currently used by FSANZ in the assessment of endosulfan residues in food. |
NRA (1998) | ADI – 0.006 mg/kg/day | As noted above from OCS (2012). |
International | ||
WHO (2011) | ADI = 0.006 mg/kg/day | No guideline is currently set in WHO (2011) as concentrations in drinking water occur well below those of health concern. However the review has noted that a health-based value of 0.02 mg/L can be derived on the basis of an ADI of 0.006 mg/kg/day derived from a 2-year dietary study in rats, supported by a 78-week study in mice, a 1 -ear study in dogs and a developmental study in rats. |
ATSDR (2000) | Oral MRL = 0.002 mg/kg/day | Chronic oral MRL based on a NOAEL of 0.18 mg/kg/day associated with liver effects in a dog study, and an uncertainty factor of 100. |
US EPA (IRIS 2012) | RfD = 0.006 mg/kg/day
| Oral RfD (last reviewed in 1994) is based on a NOAEL of 0.6/0.7 (M/F) mg/kg/day associated with kidney effects and aneurysms in a rat study, and an uncertainty factor of 100. |
Based on the available reviews, a consistent oral TRV of 0.006 mg/kg/day is available and considered suitable for the derivation of soil HILs. No inhalation or dermal data is available hence it is recommended that all intakes associated with contaminated soil be assessed on the basis of the oral TRV.
On the basis of the discussion above, the following toxicity reference values (TRVs) have been adopted for endosulfan in the derivation of HILs:

On the basis of the above the following HILs have been derived for endosulfan (refer to Appendix B for equations used to calculate the HILs and Appendix C for calculations):
HIL Scenario | HIL (mg/kg) | Percentage Contribution from Exposure Pathways | |||
Ingestion of Soil/Dust | Ingestion of Home-grown Produce | Dermal Absorption of Soil/Dust | Inhalation (dust) | ||
Residential A | 270 | 43 | -- | 57 | <1 |
Residential B | 400 | 16 | -- | 84 | <1 |
Recreational C | 340 | 27 | -- | 73 | <1 |
Commercial D | 2000 | 12 | -- | 88 | <1 |
-- Pathway not included in derivation of HIL
APVMA 2005, The Reconsideration of Approval of Active Constituent Endosulfan, Registration of Products Containing Endosulfan and their Associated Labels, Final Review Report and Regulatory Decision Review Series 2, Australian Pesticide and Veterinary Medicines Authority Canberra, Australia.
ATSDR 2000, Toxicological Profile for Endosulfan, available on website at http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=609&tid=113.
FSANZ 2003, The 20th Australian Total Diet Survey, a total diet survey of pesticide residues and contaminants, Food Standards Australia and New Zealand.
FSANZ 2011, The 23rd Australian Total Diet Study, Food Standards Australia and New Zealand.
Marshall, I & Rutherford, S 2003, ‘Health Investigation Level for Endosulfan in Soil’, presented in the proceedings of the Fifth National Workshop on the Health Risk Assessment and Management of Contaminated Sites.
NEPC 1999, Schedule B (7a), Guideline on Health-Based Investigation Levels, National Environment Protection (Assessment of Site Contamination) Measure, National Environment Protection Council, Australia.
NHMRC 2011, National water quality management strategy, Australian drinking water guidelines, National Health and Medical Research Council, Australia.
NRA 1998, The NRA Review of Endosulfan, Volume 1, Existing Chemicals Review Program, National Registration Authority for Agricultural and Veterinary Chemicals, Commonwealth of Australia, ACT, Australia.
OCS 2012, ADI List, Acceptable Daily Intakes for Agricultural and Veterinary Chemicals, current to 31 March 2012, Australian Government, Department of Health and Ageing, Office of Chemical Safety (OCS), available from: http://www.health.gov.au/internet/main/publishing.nsf/content/E8F4D2F95D616584CA2573D700770C2A/$File/ADI-apr12.pdf.
US EPA 1995, Technical Guidance Manual, Assessing Dermal Exposure from Soil, US EPA Region 3, available from: http://www.epa.gov/reg3hwmd/risk/human/info/solabsg2.htm.
US EPA (IRIS2012), data and information available from the Integrated Risk Information System, an online database, available from http://www.epa.gov/iris/.
WHO 1984, Environmental Health Criteria 40, Endosulfan, International Programme of Chemical Safety, World Health Organization, Geneva.
WHO 1998, JMPR Evaluation – Endosulfan, published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
WHO 2011, Guidelines for drinking-water quality, 4th edn, World Health Organization, Geneva, available from http://www.who.int/water_sanitation_health/dwq/chemicals/en/index.html.
Several comprehensive reviews of endrin in the environment and its toxicity to humans are available and should be consulted for more detailed information (ATSDR 1996; WHO 1992; DEH 2006). The following provides a summary of the key aspects of endrin that are relevant to the derivation of a soil HIL.
The organochlorine pesticide endrin is a white to light tan, crystalline solid with a mild chemical odour. It is relatively insoluble in water and has a low vapour pressure. Endrin is the common name for 1,2,3,4,10,10-hexachloro-6,7-epoxy-1,4, 4a,5,6,7,8,8a-octahydro-1,4-endo,endo-5,8- dimethanonaphthalene but the term is also used to describe the commercial grade insecticide which typically contains 96% endrin. Endrin is the endo,endo stereoisomer of dieldrin (WHO 1992; WHO 2011).
Endrin was manufactured as a broad-spectrum insecticide and rodenticide, which was active against a wide range of agricultural pests. It was mainly used in the cotton industry and to a lesser extent on rice, sugar cane and maize (WHO 2011). Endrin has been widely used in agriculture since the 1950s but its manufacture and use was banned under the Stockholm Convention.
Endrin use was phased out in Australia in 1987 (DEH 2006).
No previous HIL is available for endrin (NEPC 1999).
Insufficient data is available to adequately define the bioavailability of endrin hence a default approach of assuming 100% oral bioavailability has been adopted in the derivation of an HIL. It is noted that a site-specific assessment of bioavailability can be undertaken where required.
Insufficient data is available on the dermal absorption of endrin from soil. Hence the default values of 0.1 (10%) suggested by US EPA (1995) for pesticides has been adopted in the derivation of HILs.
Endrin is not considered sufficiently volatile to be of significance and inhalation exposures associated with particulates outdoors and indoors are expected to be of less significance than ingestion of soil. While likely to be negligible, potential inhalation exposures associated with dust have been considered in the HIL derived.
The few studies that are available with respect to the potential for plant uptake of endrin relate to the application of the product in solution, rather than uptake from soil. Endrin has a high Koc value (log Koc = 4.53) and low solubility in water (ATSDR 1996), suggesting that the compound is largely bound to soil particulates and is immobile in soil. For plant uptake to be significant, the chemicals must be able to partition to soil water. With respect to endrin bound to the soil, the potential for partitioning to soil water is considered to be low and hence plant uptake is considered to be negligible.
WHO (1992) provides an evaluation of exposures by the general public which are dated and relate to a period when endrin was in use. The total intake of endrin from dietary, water and air sources (noted to be dominated by dietary intakes) was estimated by WHO (1992) to be ‘far below’ the ADI adopted (0.2 µg/kg/day). Use of endrin was phased out in Australia in the late 1980s with the last product registration cancelled at the end of 1990. Hence background intakes in Australia are expected to lower than estimated by WHO. Food Standards Australia and New Zealand has not detected endrin in any sample in the 19th, 20th or 23rd food surveys (FSANZ 2003; FSANZ 2011). Hence, background intakes would be expected to be negligible. Assuming a negligible background intake is considered appropriate, based on current information.
The International Agency for Research on Cancer (IARC 1987) has classified endrin as Group 3—not classifiable, on the basis of inadequate evidence in humans and experimental animals.
It is noted that US EPA has classified endrin as Group D—not classifiable.
Insufficient data is available to indicate if endrin is carcinogenic to humans. The available data does show that endrin is not genotoxic (WHO 1992; ATSDR 1996; RIVM 2001). On the basis of the available information it is considered appropriate that a threshold doseresponse approach be adopted for endrin. The following are available from Level 1 Australian and International sources:
Source | Value | Basis/Comments |
Australian | ||
ADWG NHMRC (2011) | No evaluation available |
|
OCS (2012) | TDI = 0.0002 mg/kg/day | TDI (changed from ADI of same value in 2003) provided as endrin no longer in use in Australia. TDI adopted derived from JMPR evaluation. |
International | ||
JMPR (1970) | ADI/PTDI =0.0002 mg/kg/day | ADI first established by JMPR in 1970 based on the level that caused no toxicological effects in dietary studies in rats and dogs (NOEL of 0.025 mg/kg/day, and uncertainty factor of 100). |
WHO (2011) | PTDI =0.0002 mg/kg/day | Value available in WHO DWG based on JMPR (1970) evaluation (above). |
RIVM (2001) | TDI = 0.0002 mg/kg/day | TDI derived on basis of NOAEL of 0.025 mg/kg/day associated liver and kidney effects in a rat study, and an uncertainty factor of 100. |
ATSDR (1996) | Oral MRL = 0.0003 mg/kg/day | Chronic oral MRL based on a NOAEL of 0.025 mg/kg/day associated with CNS effects in a 2-year dog study, and an uncertainty factor of 100. |
US EPA (IRIS 2012) | RfD = 0.0003 mg/kg/day
| Oral RfD based on a NOAEL of 0.025 mg/kg/day associated with liver effects in a 2-year dog study, and an uncertainty factor of 100. |
The above evaluations have identified consistent NOAEL values and oral TRVs for the assessment of endrin intakes. Hence the current Australian TRV of 0.0002 mg/kg/day has been adopted for the derivation of soil HILs. No inhalation or dermal data is available, hence it is recommended that all intakes associated with contaminated soil be assessed on the basis of the oral TRV.
On the basis of the discussion above, the following toxicity reference values (TRVs) have been adopted for endrin in the derivation of HILs:

On the basis of the above, the following HILs have been derived for endrin (refer to Appendix B for equations used to calculate the HILs and Appendix C for calculations):
HIL Scenario | HIL (mg/kg) | Percentage Contribution from Exposure Pathways | |||
Ingestion of Soil/Dust | Ingestion of Home-grown Produce | Dermal Absorption of Soil/Dust | Inhalation (dust) | ||
Residential A | 10 | 43 | -- | 57 | <1 |
Residential B | 20 | 16 | -- | 84 | <1 |
Recreational C | 20 | 27 | -- | 73 | <1 |
Commercial D | 100 | 12 | -- | 88 | <1 |
-- Pathway not included in derivation of HIL
ATSDR 1996, Toxicological Profile for Endrin, available from: http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=617&tid=114.
DEH 2006, Stockholm Convention on Persistent Organic Pollutants, Australia’s National Implementation Plan, Department of Environment and Heritage, Commonwealth of Australia, Canberra, Australia.
FSANZ 2003, The 20th Australian Total Diet Survey, a total diet survey of pesticide residues and contaminants, Food Standards Australia and New Zealand.
FSANZ 2011, The 23rd Australian Total Diet Study, Food Standards Australia and New Zealand.
IARC 1987, Summaries & Evaluations, Endrin, International Agency for Research on Cancer, Supplement 7, p.63.
JMPR 1970, 1970 Evaluations of Some Pesticides in Food, The Monographs, Endrin, JMPR, available from: http://www.inchem.org/documents/jmpr/jmpmono/v070pr13.htm.
NEPC 1999, Schedule B (7a), Guideline on Health-Based Investigation Levels, National Environment Protection (Assessment of Site Contamination) Measure, National Environment Protection Council, Australia.
NHMRC 2011, National water quality management strategy, Australian drinking water guidelines, National Health and Medical Research Council, Australia.
OCS 2012, ADI List, Acceptable Daily Intakes for Agricultural and Veterinary Chemicals, current to 31 March 2012, Australian Government, Department of Health and Ageing, Office of Chemical Safety (OCS), available from: http://www.health.gov.au/internet/main/publishing.nsf/content/E8F4D2F95D616584CA2573D700770C2A/$File/ADI-apr12.pdf.
RIVM 2001, Re-evaluation of human-toxicological Maximum Permissible Risk levels, National Institute of Public Health and the Environment, Bilthoven, Netherlands, available from: http://www.rivm.nl/bibliotheek/rapporten/711701025.html.
US EPA 1995, Technical Guidance Manual, Assessing Dermal Exposure from Soil, US EPA Region 3, available from: http://www.epa.gov/reg3hwmd/risk/human/info/solabsg2.htm.
US EPA (IRIS 2012), data and information available from the Integrated Risk Information System, an online database, available from http://www.epa.gov/iris/.
WHO 1992, Environmental Health Criteria No 130 Endrin, International Programme of Chemical Safety, World Health Organization, Geneva.
WHO 2011, Guidelines for drinking-water quality, 4h edn, World Health Organization, Geneva, available from http://www.who.int/water_sanitation_health/dwq/chemicals/en/index.html
Several comprehensive reviews of heptachlor in the environment and its toxicity to humans are available and should be consulted for more detailed information (ATSDR 2007; WHO 1984; WHO 2006). The following provides a summary of the key aspects of heptachlor that are relevant to the derivation of a soil HIL.
Heptachlor is a polychlorinated cyclodiene insecticide that was produced and distributed from the early 1950s to the 1980s under several trade names including Heptagran®, Basaklor®, Drinox®, Soleptax®, Termide®, and Velsicol 104® (ATSDR 2007). Pure heptachlor is a white powder that smells similar to mothballs. The less pure grade commercial insecticide is tan in colour. Heptachlor is stable in light and air and does not burn easily or explode. It does not dissolve readily in water but is soluble in organic solutions (WHO 1984; ATSDR 2007).
Heptachlor is a broad-spectrum insecticide that was distributed commercially in Australia until the mid-1990s to protect against household pests such as termites. Its use in the agricultural industry to control insects in soil and seed crops was withdrawn in the late 1970s and early 1980s (NHMRC 2011). Heptachlor is a manufactured chemical and does not occur naturally. It should be noted that heptachlor is also a component of the pesticide chlordane (approximately 10%) (ATSDR 2007).
The derivation of the previous HIL (HIL A = 10 mg/kg) for heptachlor is presented by Di Marco (1993) and NEPC (1999). In summary, the HIL was derived on the basis of the following:
Insufficient data is available to adequately define the bioavailability of heptachlor hence a default approach of assuming 100% oral bioavailability has been adopted in the derivation of an HIL. It is noted, that a site-specific assessment of bioavailability can be undertaken where required.
Insufficient data is available on the dermal absorption of heptachlor from soil. Hence the default values of 0.1 (10%) suggested by US EPA (1995) for pesticides has been adopted in the derivation of HILs.
Heptachlor is not considered sufficiently volatile to be of significance and inhalation exposures associated with particulates outdoors and indoors are expected to be of less significance than ingestion of soil. While likely to be negligible, potential inhalation exposures associated with dust have been considered in the derived HIL.
The few studies that are available with respect to the potential for plant uptake of heptachlor relate to the application of the compound in solution (as a product), rather than uptake from soil. Heptachlor and heptachlor epoxide have a high Koc value (log Koc = 3.344.37) and low solubility in water (ATSDR 2007), suggesting that the compound is largely bound to the soil particulates and immobile in soil. For plant uptake to be significant, the chemicals must be able to partition to soil water. With respect to heptachlor bound to soil, the potential for partitioning to soil water is considered to be low and hence plant uptake is considered to be negligible.
For the general population, where heptachlor and heptachlor epoxide are no longer used (the last heptachlor product was cancelled by APVMA at the end of June 1997), background intakes would be expected to be primarily associated with residues in food.
Food Standards Australia and New Zealand has not detected heptachlor in any sample in the 19th, 20th or 23rd food surveys (FSANZ 2003; FSANZ 2011). Hence, background intakes would be expected to be negligible. This is consistent with reviews of background intakes estimated by Di Marco (1993), where background intakes from heptachlor (where no longer used) comprises <2% of the adopted ADI.
The International Agency for Research on Cancer (IARC 2001) has classified heptachlor as Group 2B—possibly carcinogenic to humans, based on inadequate evidence in humans and sufficient evidence in animals. It is noted that the IARC evaluation is for both chlordane and heptachlor as they are structurally related organochlorine insecticides.
It is noted that US EPA has classified heptachlor as Group B2—probable human carcinogen.
Heptachlor has been associated with carcinogenic effects but the mode of action is of prime importance for determining the most appropriate doseresponse approach to adopt for establishing an HIL. The available data (most recently reviewed by WHO 2006) does not suggest that heptachlor is genotoxic and hence a threshold approach is considered appropriate for the derivation of an HIL. Further review of heptachlor by WHO (2006) identified that non-carcinogenic (non-neoplastic) effects were observed at doses 1/20th of those where carcinogenic (neoplastic) effects were observed. Hence, use of a threshold based on non-carcinogenic effects is adequately protective of all effects (including carcinogenicity).
The following are available from Level 1 Australian and International sources:
Source | Value | Basis/Comments |
Australian | ||
ADWG (NHMRC 2011) | ADI = 0.0001 mg/kg/day | ADI referenced from JMPR evaluation (1991) and noted to be based on a NOEL of 0.025 mg/kg/day associated with liver effects from two studies using dogs, and an uncertainty factor of 200 (10 for interspecies variation, 10 for intraspecies variation and 2 for inadequacies in data base). |
OCS (2012) | TDI = 0.0005 mg/kg/day | TDI (changed from ADI of same value in 2003) provided as heptachlor no longer in use in Australia. TDI adopted derived from earlier JMPR evaluation (since updated as noted below). |
International | ||
JMPR | ADI/PTDI =0.0001 mg/kg/day | An ADI of 0.0005 mg/kg/day was estimated for heptachlor by JMPR in 1970. Review of heptachlor by JMPR in 1991 revised the ADI to 0.0001 mg/kg/day based on the level that caused no toxicological effects in studies in rats and dogs (reproduction study and 2-year dog study, NOEL of 0.025 mg/kg/day, and uncertainty factor of 200). In 1994 the JMPR converted the ADI to a PTDI with the same value. |
WHO (2011) | PTDI =0.0001 mg/kg/day | No guideline has been established by WHO as concentrations of heptachlor occur in drinking water well below those of health concern. However the review notes that a health-based value of 0.03 µg/L can be derived on the basis of a provisional TDI of 0.1 µg/kg/day, based on a NOAEL of 0.025 mg/kg/day, and uncertainty factor of 200 (as adopted in the ADWG). The review notes that water concentrations below 0.1 µg/L are generally not achievable. |
WHO (2006) | TDI = 0.0001 mg/kg/day | Based on lowest NOAEL of 0.025 mg/kg/day for histopathological liver changes from dog studies and a LOAEL/NOAEL of 0.03 mg/kg/day associated with developmental neurotoxicity and immunotoxicological studies in rats (more recent study than in dogs), and an uncertainty factor of 200. |
ATSDR (2007) | No chronic value derived | No chronic MRL was derived, however an intermediate MRL of 0.0001 mg/kg/day was derived on the basis of a LOAEL of 0.03 mg/kg/day associated with developmental immunological and neurological effects in rats (study also considered by WHO 2006), and an uncertainty factor of 300. |
US EPA (IRIS 2012) | RfD = 0.0005 mg/kg/day
| Oral RfD (last reviewed in 1987) based on a NOEL of 0.15 mg/kg/day associated with liver weight increases in a 2-year feeding study in rats and an uncertainty factor of 300. The US EPA has also derived non-threshold oral and inhalation values which are not presented here as they are not considered relevant. |
Based on the available reviews, the oral TRVs derived are generally consistent with the value adopted in the ADWG (NHMRC 2011) considered to be suitable for the derivation of soil HILs. No inhalation or dermal data is available, hence it is recommended that all intakes associated with contaminated soil be assessed on the basis of the oral TRV.
On the basis of the discussion above, the following toxicity reference values (TRVs) have been adopted for heptachlor in the derivation of HILs:

On the basis of the above, the following HILs have been derived for heptachlor (refer to Appendix B for equations used to calculate the HILs and Appendix C for calculations):
HIL Scenario | HIL (mg/kg) | Percentage Contribution from Exposure Pathways | |||
Ingestion of Soil/Dust | Ingestion of Home-grown Produce | Dermal Absorption of Soil/Dust | Inhalation (dust) | ||
Residential A | 6 | 43 | -- | 57 | <1 |
Residential B | 10 | 16 | -- | 84 | <1 |
Recreational C | 10 | 27 | -- | 73 | <1 |
Commercial D | 50 | 12 | -- | 88 | <1 |
-- Pathway not included in derivation of HIL
ATSDR 2007, Toxicological Profile for Heptachlor and Heptachlor Epoxide, US Department of Health and Human Services, ATSDR, available from http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=746&tid=135.
Di Marco, P 1993, ‘The Assessment and Management of Organochlorine Termiticides’, presented in the proceedings of the Second National Workshop on the Health Risk Assessment and Management of Contaminated Sites, Contaminated Sites Monograph Series, No. 2.
FSANZ 2003, The 20th Australian Total Diet Survey, a total diet survey of pesticide residues and contaminants, Food Standards Australia andNew Zealand.
FSANZ 2011, The 23rd Australian Total Diet Study, Food Standards Australia and New Zealand.
IARC 2001, Summaries & Evaluations, Chlordane and Heptachlor, Vol 79, p. 411, International Agency for Research on Cancer.
JMPR (various), JMPR – Monographs and Evaluations for Heptachlor, available from http://www.inchem.org/pages/jmpr.html.
NEPC 1999, Schedule B (7a), Guideline on Health-Based Investigation Levels, National Environment Protection (Assessment of Site Contamination) Measure, National Environment Protection Council, Australia.
NHMRC 2011, National water quality management strategy, Australian drinking water guidelines, National Health and Medical Research Council, Australia.
OCS 2012, ADI List, Acceptable Daily Intakes for Agricultural and Veterinary Chemicals, current to 31 March 2012, Australian Government, Department of Health and Ageing, Office of Chemical Safety (OCS), available from: http://www.health.gov.au/internet/main/publishing.nsf/content/E8F4D2F95D616584CA2573D700770C2A/$File/ADI-apr12.pdf.
US EPA 1995, Technical Guidance Manual, Assessing Dermal Exposure from Soil, US EPA Region 3, available from: http://www.epa.gov/reg3hwmd/risk/human/info/solabsg2.htm.
US EPA (IRIS 2012), data and information available from the Integrated Risk Information System, an online database, available from http://www.epa.gov/iris/.
WHO 1984, Environmental Health Criteria 38 Heptachlor, International Programme of Chemical Safety, World Health Organization, Geneva.
WHO 2006, Concise International Chemical Assessment Document (CICAD) 70, Heptachlor, available from: http://www.inchem.org/documents/cicads/cicads/cicad70.htm.
WHO 2011, Guidelines for drinking-water quality, 4th edn, World Health Organization, Geneva, available from http://www.who.int/water_sanitation_health/dwq/chemicals/en/index.html.
Several comprehensive reviews of HCB in the environment and its toxicity to humans are available and should be consulted for more detailed information (ATSDR 2002; WHO 1997). The following provides a summary of the key aspects of HCB that are relevant to the derivation of a soil HIL.
HCB is a fully chlorinated aromatic hydrocarbon. In its pure state HCB is a stable, white crystalline solid, which is insoluble in water but is soluble in fats and organic solvents. HCB has low flammability but when heated, it decomposes and emits toxic fumes. HCB was originally manufactured primarily for use as a pesticide to prevent fungal disease in seeds and grains but its use in the agricultural industry was discontinued in most countries in the mid-1960s to 1970s (WHO 1997; ATSDR 2002). HCB was also used in the manufacturing of fireworks, ammunition and synthetic rubber, and as a fluxing agent in the manufacture of aluminium. Given the concerns regarding HCB’s effect on human health and the environment, its intentional uses in commerce are limited (ATSDR 2002).
On the basis of the potential for long-range transport, persistence in air, water, soil and sediment, bioaccumulation, toxicity and ecotoxicity, HCB meets the UN-ECE Persistent Organic Pollutant (POP) criteria (UN-ECE 1998). HCB is listed under Schedule X within the National Strategy for the Management of Scheduled Waste. In addition, HCB has a National Waste Management Plan endorsed by ANZECC in 1996.
No previous HIL is available for HCB (NEPC 1999).
Insufficient data is available to adequately define the bioavailability of HCB hence a default approach of assuming 100% oral bioavailability has been adopted in the derivation of an HIL. It is noted that a site-specific assessment of bioavailability can be undertaken where required.
Insufficient data is available on the dermal absorption of HCB from soil. Hence the default value of 0.1 (10%) suggested by US EPA (1995) for pesticides has been adopted in the derivation of HILs.
HCB is not considered sufficiently volatile to be of significance and inhalation exposures associated with particulates outdoors and indoors are expected to be of less significance than ingestion of soil. While likely to be negligible, potential inhalation exposures associated with dust have been considered in the HIL derived.
The few studies that are available with respect to the potential for plant uptake of HCB relate to the application of the product in solution (as a product), or from fresh applications where some volatilisation has occurred (not considered relevant where HCB products are no longer used), rather than uptake from soil. HCB has a high Koc value (log Koc = 3.596.08) and low solubility in water (ATSDR 2002), suggesting that the compound is largely bound to the soil particulates and is immobile in soil. For plant uptake to be significant, the chemicals must be able to partition to soil water. With respect to HCB bound to the soil, the potential for partitioning to soil water is considered to be low and hence plant uptake is considered to be negligible.
For the general population, away from areas where significant stores of HCB waste have been identified (refer to HCB Waste Management Plan, ANZECC 1996), background intakes would be expected to be primarily associated with residues in food.
Food Standards Australia and New Zealand has not detected HCB in any sample in the 18th, 19th or 20th food surveys (FSANZ 2003). WHO (1997) calculated that the total background intake by an adult of HCB is between 0.0004 and 0.003 µg/kg body weight per day, mostly derived from dietary exposures. This intake is essentially negligible compared to the recommended oral TRV. Hence, background intakes would be expected to be negligible. This is consistent with reviews of background intakes estimated by RIVM (2001).
The International Agency for Research on Cancer (IARC 2001) has classified HCB as Group 2B—possibly carcinogenic to humans, based on inadequate evidence in humans and sufficient evidence in animals.
HCB has been classified as a probable human carcinogen (Category B2) by US EPA on the basis of the induction of tumours in the liver, thyroid and kidney in three rodent species following oral exposure.
HCB has been associated with carcinogenic effects however; the mode of action is of prime importance for determining the most appropriate doseresponse approach to adopt for establishing an HIL. The available data (WHO DWG 2011; WHO 1997; RIVM 2001) shows that the weight of evidence does not suggest that HCB is genotoxic and hence a threshold approach is considered appropriate for the derivation of an HIL. Further review of HCB by WHO (1997) considered the derivation of a threshold TDI based on both non-neoplastic and neoplastic effects (similar threshold values), hence appropriate threshold values are available that adequately address neoplastic (carcinogenic) effects.
The following are available from Level 1 Australian and International sources:
Source | Value | Basis/Comments |
Australian | ||
ADWG (NHMRC 2011) | No evaluation available |
|
OCS (2012) | No evaluation available |
|
International | ||
JMPR | ADI =0.0006 mg/kg/day | An ADI of 0.0006 mg/kg/day was estimated for HCB by JMPR in 1969 (reaffirmed in 1974) based on a dietary study in rats. |
WHO (2011) | TDI = 0.00016 mg/kg/day | No guideline has been established by WHO, as concentrations of HCB occur in drinking water well below those of health concern. However the review conducted (in 2003) has indicated a health-based guideline of 0.0005 to 0.001 mg/L can be derived on the basis of a linear multistage low-dose extrapolation model or a TDI of 0.00016 mg/kg/day. |
WHO (1997) | TDI = 0.00016 mg/kg/day | TDI presented is the lowest TDI derived on the basis of non-neoplastic and neoplastic effects. A TDI of 0.00017 mg/kg/day was derived on the basis of a NOEL of 0.05 mg/kg/day associated hepatic effects in pigs and rats, and an uncertainty factor of 200 (the evaluation presented included consideration of the study used by ATSDR in the derivation of the chronic MRL). The lower TDI presented was derived on the basis of a Tumorigenic Dose (TD5) associated with a 5% excess incidence in tumours in experimental animals. The TDI was derived on the basis of results from a 2-generation study in rats where the TD5 values ranged from 0.81 mg/kg/day to 2.01 mg/kg/day. The lower value associated with neoplastic effects in the liver was adopted with an uncertainty factor of 5000 to derive the TDI of 0.00016 mg/kg/day. It is noted that review also derived a TDI for non-neoplastic effects of 0.00017 mg/kg/day based on a NOEL of 0.05 mg/kg/day based on hepatic effects in a subchronic study in pigs and a chronic study in rats. This evaluation considered the same studies as ATSDR and US EPA and the non-neoplastic TDI is similar to that derived for neoplastic effects. |
ATSDR (2002) | Oral MRL = 0.00005 mg/kg/day | Chronic oral MRL derived on the basis of a LOAEL of 0.016 mg/kg/day for peribiliary lymphocytosis and fibrosis of the liver in a 2-generation study on rats, and an uncertainty factor of 300. The ATSDR review has considered the same study as the US EPA (Arnold et al. 1985), however they have applied slightly different uncertainty factors to the NOAEL. |
US EPA (IRIS 2012) | RfD = 0.0008 mg/kg/day
| Oral RfD (last reviewed in 1988) based on a NOAEL of 0.08 mg/kg/day associated with liver effects in a rat study, and an uncertainty factor of 100. The US EPA has also derived non-threshold oral and inhalation values which are not presented here as they are not considered relevant. |
Based on the available reviews presented above, a range of oral TRVs is available. The oral TRV adopted by WHO (1997) is relevant to both neoplastic and non-neoplastic effects (note the ATSDR and US EPA threshold values relate to non-neoplastic effects only) and is therefore considered suitable and appropriate for use in the derivation of soil HILs. No inhalation or dermal data are available, hence it is recommended that all intakes associated with contaminated soil be assessed on the basis of the oral TRV.
On the basis of the discussion above, the following toxicity reference values (TRVs) have been adopted for HCB in the derivation of HILs:
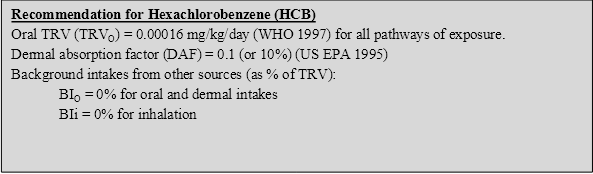
On the basis of the above, the following HILs have been derived for HCB (refer to Appendix B for equations used to calculate the HILs and Appendix C for calculations):
HIL Scenario | HIL (mg/kg) | Percentage Contribution from Exposure Pathways | |||
Ingestion of Soil/Dust | Ingestion of Home-grown Produce | Dermal Absorption of Soil/Dust | Inhalation (dust) | ||
Residential A | 10 | 43 | -- | 57 | <1 |
Residential B | 15 | 16 | -- | 84 | <1 |
Recreational C | 10 | 27 | -- | 73 | <1 |
Commercial D | 80 | 12 | -- | 88 | <1 |
-- Pathway not included in derivation of HIL
ANZECC 1996, Hexachlorobenzene Water Management Plan, available from http://www.environment.gov.au/settlements/chemicals/scheduled-waste/index.html#hcbs.
Arnold, DL, Moodie, CA, Charbonneau, SM, Grice, HC, McGuire, PF, Bryce, FR, Collins, BT, Zwadizka, ZZ, Krewski, DR, Nera, EA & Munro, IC 1985, ‘Long-term toxicity of hexachlorobenzene in the rat and the effect of dietary vitamin A’, Food Chem Toxicol, vol. 23(9), pp. 779793.
ATSDR 2002, Toxicological Profile for Hexachlorobenzene, US Department of Health and Human Services, ATSDR, available from http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=627&tid=115.
FSANZ 2003, The 20th Australian Total Diet Survey, a total diet survey of pesticide residues and contaminants, Food Standards Australia and New Zealand.
IARC 2001, Summaries & Evaluations, Hexachlorobenzene, vol. 79, p. 493, International Agency for Research on Cancer.
JMPR (various), JMPR – Monographs and Evaluations for Hexachlorobenzene, available from http://www.inchem.org/pages/jmpr.html.
NEPC 1999, Schedule B (7a), Guideline on Health-Based Investigation Levels, National Environment Protection (Assessment of Site Contamination) Measure, National Environment Protection Council, Australia.
NHMRC 2011, National water quality management strategy, Australian drinking water guidelines, National Health and Medical Research Council, Australia.
RIVM 2001, Re-evaluation of human-toxicological Maximum Permissible Risk levels, National Institute of Public Health and the Environment, Bilthoven, Netherlands, available from: http://www.rivm.nl/bibliotheek/rapporten/711701025.html.
UN-ECE 1998, Protocol on Persistent Organic Pollutants, United Nations Economic Commission for Europe.
US EPA 1995, Technical Guidance Manual, Assessing Dermal Exposure from Soil. US EPA Region 3, available from: http://www.epa.gov/reg3hwmd/risk/human/info/solabsg2.htm.
US EPA (IRIS 2012), data and information available from the Integrated Risk Information System, an online database, available from http://www.epa.gov/iris/.
WHO 1997, Environmental Health Criteria 195, Hexachlorobenzene, International Programme On Chemical Safety, United Nations Environment Programme, International Labour Organisation, World Health Organization.
WHO 2011, Guidelines for drinking-water quality, 4th edn, World Health Organization, Geneva, available from http://www.who.int/water_sanitation_health/dwq/chemicals/en/index.html.
Several comprehensive reviews of methoxychlor in the environment and its toxicity to humans are available and should be consulted for more detailed information (ATSDR 2002; ATSDR 2009; JMPR 1977; OEHHA 1999). The following provides a summary of the key aspects of methoxychlor that are relevant to the derivation of a soil HIL.
Methoxychlor does not occur naturally in the environment. Pure methoxychlor is a pale yellow powder that has a slightly fruity or musty odour. It does not readily evaporate or dissolve in water (ATSDR 2002).
Methoxychlor is a manufactured chemical used for controlling insects. The chemical is effective against flies, mosquitoes and cockroaches and is used on agricultural crops and livestock, in animal feed barns and in grain storage bins. Some pesticide products that contain methoxychlor are used for controlling insects in gardens or on household pets (ATSDR 2002).
It is noted that the only methoxychlor product registration in Australian was cancelled in mid-1987 (information available from APVMA).
No previous HIL is available for methoxychlor (NEPC 1999).
Insufficient data is available to adequately define the bioavailability of methoxychlor hence a default approach of assuming 100% oral bioavailability has been adopted in the derivation of an HIL. It is noted that a site-specific assessment of bioavailability can be undertaken where required.
Insufficient data is available on the dermal absorption of methoxychlor from soil. Hence the default value of 0.1 (10%) suggested by US EPA (1995) for pesticides has been adopted in the derivation of HILs.
Methoxychlor is not considered sufficiently volatile to be of significance and inhalation exposures associated with particulates outdoors and indoors are expected to be of less significance than ingestion of soil. While likely to be negligible, potential inhalation exposures associated with dust have been considered in the HIL derived.
There is no information regarding the plant uptake of methoxychlor following its application as a pesticide (ATSDR 2002). Methoxychlor has a high Koc value (log Koc = 4.9) and low solubility in water (ATSDR 2002), suggesting that the compound is largely bound to soil particulates and is immobile in soil. For plant uptake to be significant, the chemicals must be able to partition to soil water. With respect to methoxychlor bound to the soil, the potential for partitioning to soil water is considered to be low and hence plant uptake is considered to be negligible.
No data is available regarding background concentrations of methoxychlor in Australia. The 23rd Australian Total Diet Study (FSANZ 2011) did not detect methoxychlor in any products sampled and tested. US data is available for dietary intakes, but as methoxychlor is not used in Australia and is generally not persistent in the environment, background intakes can be assumed to be essentially negligible.
The International Agency for Research on Cancer (IARC 1987) has classified methoxychlor as Group 3—not classifiable.
It is noted that US EPA has classified methoxychlor as Class D—not classifiable.
Review of methoxychlor by the WHO (2011) and ATSDR (2002) indicates that recent data (considered inadequate to be conclusive) suggests some carcinogenic potential for methoxychlor for liver and testis in mice, which may be due to the hormonal activity of progestogenic metabolites and may therefore have a threshold. It is noted that methoxychlor is a suspected endocrine disruptor. The review undertaken by ASTDR (2002) considered available genotoxicity studies, with most showing negative results. Hence the genotoxic potential of methoxychlor appears to be negligible (WHO 2011). On this basis, it is appropriate that a threshold approach be adopted. The following are available from Level 1 Australian and International sources:
Source | Value | Basis/Comments |
Australian | ||
ADWG (NHMRC 2011) | TDI = 0.09 mg/kg/day | A health-based guideline of 0.3 mg/L is presented in the ADWG (NHMRC 2011). While no further information is available, based on the assumptions adopted in the ADWG (10% intake from drinking water, 2 L/day and 70 kg body weight), the guideline was derived from a TDI of 0.09 mg/kg/day. |
OCS (2012) | TDI = 0.1 mg/kg/day | TDI (set in 2003, changed from the ADI of the same value), noted to be based on the JMPR evaluation. It is noted that methoxychlor is no longer used in Australia and hence the TDI is maintained to address potential dietary intakes. |
International | ||
JMPR (1977) | ADI =0.1 mg/kg/day | The ADI of 0.1 mg/kg/day was established in 1963, reviewed and confirmed in 1965 and 1977 based on a NOEL of 10 mg/kg/day in a dietary rat study, and an uncertainty factor of 100. |
WHO (2011) | TDI = 0.005 mg/kg/day | A guideline of 0.02 mg/L (last reviewed in 2003) has been derived on the basis of a TDI of 0.005 mg/kg/day from a NOAEL of 5 mg/kg/day associated with a teratology study in rabbits, and an uncertainty factor of 1000 (including a 10 fold factor to address concern for threshold carcinogenicity and the limited database). Methoxychlor is noted to be included in the rolling revision of the WHO DWG. No revised evaluations are currently available. |
ATSDR (2002) | No chronic oral MRL derived | No chronic MRL derived, however an intermediate duration MRL of 0.005 mg/kg/day was derived. |
OEHHA (1999) | RfD = 0.005 mg/kg/day
| A public health goal (drinking water guideline) of 0.03 mg/L was derived on the basis of an RfD of 0.005 mg/kg/day derived from a LOAEL of 5 mg/kg/day associated with developmental effects in female rats, and an uncertainty factor of 1000. |
US EPA (IRIS 2012) | RfD = 0.005 mg/kg/day
| Oral RfD (last reviewed in 1990) based on a NOEL of 5 mg/kg/day associated with reproductive effects in rabbits, and an uncertainty factor 1000. |
Based on the available data above, essentially two threshold values are available, though they are significantly different. The ADI available from JMPR has not been reviewed since 1977 and has not considered the studies used in subsequent evaluations in WHO (2011) and presented by the OEHHA, ATSDR and US EPA. While the older JMPR has been adopted by OCS (2012) and NHMRC (2011), the lower value presented in the current WHO DWG is more technically sound and has been adopted for the purpose of deriving soil HILs. No inhalation or dermal data are available, hence it is recommended that all intakes associated with contaminated soil be assessed on the basis of the oral TRV.
On the basis of the discussion above, the following toxicity reference values (TRVs) have been adopted for methoxychlor in the derivation of HILs:

On the basis of the above the following HILs have been derived for methoxychlor (refer to Appendix B for equations used to calculate the HILs and Appendix C for calculations):
HIL Scenario | HIL (mg/kg) | Percentage Contribution from Exposure Pathways | |||
Ingestion of Soil/Dust | Ingestion of Home-grown Produce | Dermal Absorption of Soil/Dust | Inhalation (dust) | ||
Residential A | 300 | 43 | -- | 57 | <1 |
Residential B | 500 | 16 | -- | 84 | <1 |
Recreational C | 400 | 27 | -- | 73 | <1 |
Commercial D | 2500 | 12 | -- | 88 | <1 |
-- Pathway not included in derivation of HIL
ATSDR 2002, Toxicological Profile for Methoxychlor, Agency for Toxic Substances and Disease Registry, available from http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=778&tid=151.
ATSDR 2009, Addendum to the Toxicological Profile for Methoxychlor, Agency for Toxic Substances and Disease Registry, available from http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=778&tid=151.
FSANZ 2011, The 23rd Australian Total Diet Study, Food Standards Australia and New Zealand.
IARC 1987, Summaries & Evaluations, Methoxychlor, Suppl 7, p.66, International Agency for Research on Cancer.
JMPR 1977, Methoxychlor, Evaluation of the Joint Meeting on Pesticide Residue, available at http://www.inchem.org/documents/jmpr/jmpmono/v077pr37.htm.
NEPC 1999, Schedule B (7a), Guideline on Health-Based Investigation Levels, National Environment Protection (Assessment of Site Contamination) Measure, National Environment Protection Council, Australia.
NHMRC 2011, National water quality management strategy, Australian drinking water guidelines, National Health and Medical Research Council, Australia.
OCS 2012, ADI List, Acceptable Daily Intakes for Agricultural and Veterinary Chemicals, current to 31 March 2012, Australian Government, Department of Health and Ageing, Office of Chemical Safety (OCS), available from: http://www.health.gov.au/internet/main/publishing.nsf/content/E8F4D2F95D616584CA2573D700770C2A/$File/ADI-apr12.pdf.
OEHHA 1999, Public Health Goal for Methoxychlor in Drinking Water, Office of Environmental Health Hazard Assessment, California Environmental Protection Agency.
US EPA 1995, Technical Guidance Manual, Assessing Dermal Exposure from Soil, US EPA Region 3, available from: http://www.epa.gov/reg3hwmd/risk/human/info/solabsg2.htm.
US EPA (IRIS 2012), data and information available from the Integrated Risk Information System, an online database, available from http://www.epa.gov/iris/.
WHO 2011, Guidelines for drinking-water quality, 4th edn, World Health Organization, Geneva, available from http://www.who.int/water_sanitation_health/dwq/chemicals/en/index.html.
Several comprehensive reviews of mirex in the environment and its toxicity to humans are available and should be consulted for more detailed information (ATSDR 1995; WHO 1984; US EPA 2003). The following provides a summary of the key aspects of mirex that are relevant to the derivation of a soil HIL.
Mirex is the common name for the compound 1,1a,2,2,3,3a,4,5,5,5a,5b,6-dodecachloroocta-hydro-1,3,4-metheno-1H-cyclobuta[c,d]pentalene. Mirex can also be used to describe an insecticide containing the compound as its active ingredient. The mirex compound is an odourless, white, crystalline solid. It is virtually insoluble in water, has a low vapour pressure and does not burn easily (WHO 1984; ATSDR 1995). Mirex is very stable and does not react with acids (sulfuric, nitric or hydrochloric), bases, chlorine or ozone (WHO 1984).
Until recently, mirex was used extensively in the Northern Territory to control giant termite populations. An exemption was granted under the Stockholm Convention for the continued use of mirex for giant termite control but the compound was completely banned in Australia in 2007 following the development of suitable alternative treatments (DEH 2006; APVMA 2007). Mirex was historically used as a pesticide to control fire ants, mostly in the south-eastern part of the United States. It was also used as a flame retardant in plastics, rubber, paint, paper and electrical goods and as a smoke generating compound when mixed with zinc oxide and aluminium (WHO 1984; ATSDR 1995).
No previous HIL is available for mirex (NEPC 1999).
Insufficient data is available to adequately define the bioavailability of mirex hence a default approach of assuming 100% oral bioavailability has been adopted in the derivation of an HIL. It is noted that a site-specific assessment of bioavailability can be undertaken where required.
Insufficient data is available on the dermal absorption of mirex from soil. Hence the default value of 0.1 (10%) suggested by US EPA (1995) for pesticides has been adopted in the derivation of HILs.
Mirex is not considered sufficiently volatile to be of significance and inhalation exposures associated with particulates outdoors and indoors are expected to be of less significance than ingestion of soil. While likely to be negligible, potential inhalation exposures associated with dust have been considered in the HIL derived.
Mirex is noted to bioaccumulate in terrestrial plants (ATSDR 1995). Limited data shows plant uptake of mirex when applied as a pesticide, however no data is available on plant uptake from soil with residual contamination of mirex. Mirex has a high Koc value (log Koc = 3.7) and low solubility in water (ATSDR 1995), suggesting that the compound is largely bound to soil particulates and is immobile in soil. For plant uptake to be significant, the chemicals must be able to partition to soil water. With respect to mirex bound to the soil, the potential for partitioning to soil water is considered to be low and hence plant uptake is considered to be negligible.
No data is available regarding background concentrations of mirex in Australia. Given the chemical’s persistence in the environment, dietary intakes (particularly seafood) are expected to be of most significance for the general population. Mirex has not been detected in any food products evaluated in the 23rd Australian Total Diet Survey (FSANZ 2011). Limited data reported from the US in WHO (1984) suggest intakes of mirex from fish consumption ranged from 0.13 µg/day to 0.39 µg/day. These intakes are low however, if the upper value were considered this may comprise up to 10% of the recommended oral TRV. Mirex use in Australia in the past decade was limited (only in the NT) prior to final phase-out in 2007. Hence, background intakes in Australia are expected to be less than reported in the US. It is therefore reasonable to consider background intakes as essentially negligible.
The International Agency for Research on Cancer (IARC 1987) has classified mirex as Group 2B—possibly carcinogenic to humans.
It is noted, that the current information from US EPA (IRIS 2012) notes that US EPA has not classified mirex. A draft review of mirex (US EPA 2003) identified mirex as likely to be carcinogenic to humans based on consistent findings of hepatic carcinogenic responses and less consistent findings of tumours in other tissues in several dietary studies in rats and mice. The human relevance of the animal evidence of carcinogenicity is assumed in the absence of adequate human data or mechanistic data to indicate that the mode of carcinogenic action in animals is not relevant to humans.
There is some evidence that mirex is carcinogenic, based on studies in experimental rats and mice (WHO 1984). More recent review of mirex by US EPA (2003) indicates that the mechanism by which mirex causes non-neoplastic and neoplastic lesions in the liver is poorly understood. Mirex has not been genotoxic in numerous short-term in vitro and a few in vivo tests (also noted by WHO 1984), leading to the hypothesis that tumorigenic responses to mirex in the liver do not directly involve a genotoxic mechanism and may involve proliferation of cells initiated spontaneously, or by some other agent, to become tumours. Review by ATSDR (1995) suggested that, based on weight of evidence, mirex is a probable tumour promoter.
On this basis, it is appropriate that a threshold approach be adopted. The following are available from Level 1 Australian and International sources:
Source | Value | Basis/Comments |
Australian | ||
ADWG (NHMRC 2011) | No evaluation available |
|
OCS (2012) | No evaluation available |
|
International | ||
JMPR/WHO (1984) | No ADI established | No ADI was established by WHO (1984) or by JMPR. |
WHO (2011) | No evaluation available | No guideline established as mirex is listed as excluded from the DWG as it is unlikely to occur in drinking-water. |
ATSDR (1995) | MRL = 0.0008 mg/kg/day | Chronic oral MRL based on a NOAEL of 0.05 mg/kg/day for dose-dependent liver changes in a chronic duration rat study, and an uncertainty factor of 100. |
US EPA (IRIS 2012) | RfD = 0.0002 mg/kg/day
| Oral RfD currently within IRIS (last reviewed in 1992) based on a NOAEL of 0.07 mg/kg/day associated with liver effects in a chronic duration rat study, and an uncertainty factor of 300. Mirex is currently being reassessed by the US EPA with a draft (2003) review deriving an oral RfD of 0.0005 mg/kg/day derived using a benchmark approach based on a LED10 of 0.15 mg/kg/day associated with liver effects in male and female rats, and an uncertainty factor of 300. A non-threshold (linear) assessment was also presented. This draft review has not been finalised as at March 2012. |
Limited quantitative data is available for mirex, with the few values available within the same order or magnitude. As there are so few evaluations available, the lower value currently available on IRIS is considered reasonable. No inhalation or dermal data are available, hence it is recommended that all intakes associated with contaminated soil be assessed on the basis of the oral TRV.
On the basis of the discussion above the following toxicity reference values (TRVs) have been adopted for mirex in the derivation of HILs:

On the basis of the above, the following HILs have been derived for mirex (refer to Appendix B for equations used to calculate the HILs and Appendix C for calculations):
HIL Scenario | HIL (mg/kg) | Percentage Contribution from Exposure Pathways | |||
Ingestion of Soil/Dust | Ingestion of Home-grown Produce | Dermal Absorption of Soil/Dust | Inhalation (dust) | ||
Residential A | 10 | 43 | -- | 57 | <1 |
Residential B | 20 | 16 | -- | 84 | <1 |
Recreational C | 20 | 27 | -- | 73 | <1 |
Commercial D | 100 | 12 | -- | 88 | <1 |
-- Pathway not included in derivation of HIL
APVMA 2007, Special Gazette No S27, Monday 5th February 2007, APVMA Notice Regarding Agricultural Chemical Products Mirant, Commonwealth of Australia, Canberra, Australia.
ATSDR 1995, Toxicological Profile for Mirex and Chlordecone, Agency for Toxic Substances and Disease Registry, available from http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=643&tid=118.
DEH 2006, Stockholm Convention on Persistent Organic Pollutants, Australia’s National Implementation Plan, Commonwealth of Australia, Canberra, Australia.
FSANZ 2011, The 23rd Australian Total Diet Study, Food Standards Australia and New Zealand.
IARC 1987, Summaries & Evaluations, Mirex, Suppl. 7, p.66, International Agency for Research on Cancer.
NEPC 1999, Schedule B (7a), Guideline on Health-Based Investigation Levels, National Environment Protection (Assessment of Site Contamination) Measure, National Environment Protection Council, Australia.
NHMRC 2011, National water quality management strategy, Australian drinking water guidelines, National Health and Medical Research Council, Australia.
US EPA 1995, Technical Guidance Manual, Assessing Dermal Exposure from Soil, US EPA Region 3, available from: http://www.epa.gov/reg3hwmd/risk/human/info/solabsg2.htm.
US EPA (IRIS 2012), data and information available from the Integrated Risk Information System, an online database, available from http://www.epa.gov/iris/.
US EPA 2003, Toxicological Review of Mirex, in support of Summary Information on the Integrated Risk Information System (IRIS), External Review Draft.
WHO 1984, Environmental Health Criteria 44 Mirex, International Programme of Chemical Safety, World Health Organization, Geneva.
WHO 2011, Guidelines for drinking-water quality, 4th edn, World Health Organization, Geneva, available from http://www.who.int/water_sanitation_health/dwq/chemicals/en/index.html.
Several comprehensive reviews of toxaphene in the environment and its toxicity to humans are available and should be consulted for more detailed information (ATSDR 1996; IARC 2001). The following provides a summary of the key aspects of toxaphene that are relevant to the derivation of a soil HIL.
Toxaphene is a mixture of over 670 compounds produced via the chlorination of camphenes from pine resins. Toxaphene is the active ingredient in a class of insecticides produced under a number of trade names including the following: Alltox, Attac, Camphechlor, chlorinated camphene, Compound 3956, Huilex, Melipax, Motox, Strobane-T, Texadust and Toxakil (ATSDR 1996). In its original form, toxaphene is a yellow, waxy solid with a chlorine or turpentine-like odour. It is highly insoluble in water but readily soluble in aromatic hydrocarbons. It is not flammable and evaporates slowly when in its solid form or when mixed with liquids (ATSDR 1996; IARC 2001).
Toxaphene was manufactured and used as a non-systemic, contact insecticide from the late 1940s. Its use was banned in Australia (since 1987) and much of the world in the 1980s; however, toxaphene is still used (to some degree) in some developing regions of the world including Africa, Asia and South America (ATSDR 1996). Insecticide products containing toxaphene were primarily used on cotton, cereal grains, fruits, nuts and vegetables and were also used to control lice, ticks and mites in livestock and fish populations in water bodies (ATSDR 1996; IARC 2001). Toxaphene was never used in Australia, with any registered products cancelled in 1987.
No previous HIL is available for toxaphene (NEPC 1999).
Insufficient data is available to adequately define the bioavailability of toxaphene hence a default approach of assuming 100% oral bioavailability has been adopted in the derivation of an HIL. It is noted that a site-specific assessment of bioavailability can be undertaken where required.
Insufficient data is available on the dermal absorption of toxaphene from soil. Hence the default value of 0.1 (10%) suggested by US EPA (1995) for pesticides has been adopted in the derivation of HILs.
Toxaphene is not considered sufficiently volatile to be of significance and inhalation exposures associated with particulates outdoors and indoors are expected to be of less significance than ingestion of soil. While likely to be negligible, potential inhalation exposures associated with dust have been considered in the HIL derived.
Limited data is available on the potential for plant uptake of toxaphene. ATSDR (1996) notes that toxaphene is not expected to be available to humans via ingestion of plants unless they have been recently treated with the mixture.
Toxaphene has a high Koc value (log Koc = 2.45) and very low solubility in water (ATSDR 1996), suggesting that the compound is largely bound to soil particulates and is immobile in soil. For plant uptake to be significant, the chemicals must be able to partition to soil water.
With respect to toxaphene bound to the soil, the potential for partitioning to soil water is considered to be low and hence plant uptake is considered to be negligible.
No data is available regarding background concentrations of toxaphene in Australia. Given the chemical’s persistence in the environment and that it is no longer used in Australia, dietary intakes of residues are expected to be of most significance for the general population. Toxaphene was not included in the Australian Total Diet Surveys. Limited data reported from the US (ATSDR 1996) suggests intakes from dietary sources (based on data from 19861991) are approximately 0.007 µg/kg/day for adults and 0.0224 µg/kg/day for children aged 2 years. These intakes are less than 10% of the recommended threshold TRV and, given that the compound is not used in Australia (and has not been used in 1987), potential background intakes are expected to be lower than estimated in the US. As no Australian data is available to confirm this assumption, a conservative approach has been adopted where background intakes have been assumed to comprise 10% of the adopted TRV.
The International Agency for Research on Cancer (IARC 2001) has classified toxaphene as Group 2B—possibly carcinogenic to humans.
It is noted that US EPA has classified toxaphene as Group B2—probable human carcinogen. The evaluation is based on increased hepatocellular adenomas and carcinomas in mice and increased thyroid tumours in rats.
Toxaphene induces hepatocellular adenomas and carcinomas in mice, thyroid follicular-cell adenomas and carcinomas in both sexes of rats, and pituitary adenomas in female rats. However, the available human data did not indicate a significant increase in cancer risk associated with exposure to toxaphene (IARC 2001). The mechanism underlying the carcinogenic effect is at present unclear. IARC (2001) notes that some in vitro tests for genotoxicity were positive, however due to limitations in the available database it cannot be concluded if toxaphene has genotoxic potential in vivo or not. Review by ATSDR (1996) suggests that toxaphene may be genotoxic. Review by ATSDR (1996) suggests that while organochlorines in general induce carcinogenic effects via an epigenetic mechanism rather than a genotoxic mechanism, the available data for toxaphene does not suggest that it meets all the criteria for an epigenetic carcinogen. Hence, ATSDR concludes that toxaphene may induce carcinogenicity via an epigenetic and genotoxic mechanism. The available data shows that toxaphene may be weakly genotoxic though there is insufficient data available to suggest that a non-threshold approach is relevant.
Few quantitative toxicity values are available for toxaphene with the following available from Level 1 Australian and International sources:
Source | Value | Basis/Comments |
Australian | ||
ADWG | No evaluation available |
|
OCS (2012) | No evaluation available |
|
International | ||
JMPR/WHO | No ADI established | NO ADI was established by WHO or by JMPR for toxaphene or campheclor. |
WHO (2011) | No evaluation available |
|
ATSDR (1996) | No chronic MRLs derived | No chronic duration MRLs derived, however ATSDR has derived an intermediate duration oral MRL of 0.001 mg/kg/day based on hepatic effects. |
OEHHA (2003) | RfD = 0.00035 mg/kg/day
| RfD derived to assess non-carcinogenic effects. Value based on a NOAEL of 0.35 mg/kg/day associated with slight hepatic changes in rats, and an uncertainty factor of 1000. An oral slope factor has also been derived, however it is not considered to be relevant for this evaluation. |
US EPA (IRIS 2012) | No threshold value calculated
| The US EPA review has not derived any threshold TRVs. The only values derived are non-threshold oral and inhalation values not considered to be relevant for this evaluation. Oral slope factor (last reviewed in 1991) derived on the basis of hepatocellular carcinomas and neoplastic nodules in a dietary study in mice and a linearised multistage model. Inhalation unit risk based on route extrapolation from the derived oral slope factor. No threshold values available. |
Limited quantitative data is available for toxaphene with the only threshold value available from OEHHA. No inhalation or dermal-specific data is available and hence the oral value has been used to assess intakes derived from all pathways of exposure.
On the basis of the discussion above, the following toxicity reference values (TRVs) have been adopted for toxaphene in the derivation of HILs:

On the basis of the above, the following HILs have been derived for toxaphene (refer to Appendix B for equations used to calculate the HILs and Appendix C for calculations):
HIL Scenario | HIL (mg/kg) | Percentage Contribution from Exposure Pathways | |||
Ingestion of Soil/Dust | Ingestion of Home-grown Produce | Dermal Absorption of Soil/Dust | Inhalation (dust) | ||
Residential A | 20 | 43 | -- | 57 | <1 |
Residential B | 30 | 16 | -- | 84 | <1 |
Recreational C | 30 | 27 | -- | 73 | <1 |
Commercial D | 160 | 12 | -- | 88 | <1 |
-- Pathway not included in derivation of HIL
ATSDR 1996, Toxicological Profile for Toxaphene, Agency for Toxic Substances and Disease Registry. Available from http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=548&tid=99.
IARC 2001, Summaries and Evaluations, Toxaphene, vol. 79, International Agency for Research on Cancer.
NEPC 1999, Schedule B (7a), Guideline on Health-Based Investigation Levels, National Environment Protection (Assessment of Site Contamination) Measure, National Environment Protection Council, Australia.
NHMRC 2011, National water quality management strategy, Australian drinking water guidelines, National Health and Medical Research Council, Australia.
OEHHA 2003, Public Health Goals for Chemicals in Drinking Water, Toxaphene, Office of Environmental Health Hazard Assessment (OEHHA), September 2003.
US EPA 1995, Technical Guidance Manual, Assessing Dermal Exposure from Soil, US EPA Region 3, available from: http://www.epa.gov/reg3hwmd/risk/human/info/solabsg2.htm.
US EPA (IRIS 2012), data and information available from the Integrated Risk Information System, an online database, available from http://www.epa.gov/iris/.
WHO 2011, Guidelines for drinking-water quality, 4th edn, World Health Organization, Geneva, available from http://www.who.int/water_sanitation_health/dwq/chemicals/en/index.html.
ADI | acceptable daily intake |
ADWG | Australian Drinking Water Guidelines |
AI | adequate intake |
ANZECC | Australia and New Zealand Environment and Conservation Council |
APVMA | Australian Pesticides and Veterinary Medicines Authority |
ATDS | Australian Total Diet Survey |
ATSDR | Agency for Toxic Substances and Disease Registry |
BA | bioavailability |
BI | background intake |
BMD | benchmark dose |
BMDL | Benchmark dose lower confidence limit |
CCME | Canadian Council of Ministers of the Environment |
CICAD | Concise International Chemicals Assessment Document |
CNS | central nervous system |
DAF | dermal absorption factor |
DW | dry weight |
EA | Environment Agency (England and Wales) |
EHC | Environmental Health Criteria |
EPA | Environment Protection Authority |
FSANZ | Food Standards Australia and New Zealand |
GAF | gastrointestinal absorption factor |
HCB | hexachlorobenzene |
HEC | human equivalent concentration |
HED | human equivalent dose |
HIARC | Hazard Identification Assessment Review Committee |
HIL | health investigation level |
HSDB | Hazardous Substances Data Bank |
HSL | health screening level |
IARC | International Agency for Research on Cancer |
IEUBK | Integrated exposure uptake biokinetic model |
IRIS | Integrated Risk Information System |
JECFA | Joint FAO/WHO Expert Committee on Food Additives |
JMPR | WHO/FAO Joint Meeting on Pesticide Residues |
LOAEL | lowest observed adverse effect level |
LOEL | lowest observed effect level |
MF | modifying factor |
MOA | mode (or mechanism) of action |
MRL | minimal risk level |
NEPC | National Environment Protection Council |
NEPM | National Environment Protection Measure |
NHMRC | National Health and Medical Research Council |
NOAEL | no observable adverse effect level |
NOEL | no observable effect level |
NSW DECC | New South Wales Department of Environment and Climate Change |
OCS | Office of Chemical Safety |
POP | persistent organic pollutant |
PTDI | provisional tolerable daily intake |
PTMI | provisional tolerable monthly intake |
PTWI | provisional tolerable weekly intake |
RAIS | Risk Assessment Information System |
RDI | recommended daily intake |
REL | reference exposure level |
RfC | reference concentration |
RfD | reference dose |
RME | reasonable maximum exposure |
SF | slope factor |
TC | tolerable concentration |
TD | tumorigenic dose |
TDI | tolerable daily intake |
TRV | toxicity reference value |
UF | uncertainty factor |
UL | upper limit |
UR | unit risk |
US EPA | United States Environmental Protection Agency |
WHO | World Health Organization |
WHO DWG | World Health Organization Drinking Water Guidelines |