
![]()
![]()


![]()
![]()

 Page
Page
1 2,4,5-T
1.1 General
1.2 Previous HIL
1.3 Significance of Exposure Pathways
1.3.1 Oral Bioavailability
1.3.2 Dermal absorption
1.3.3 Inhalation of Dust
1.3.4 Plant Uptake
1.3.5 Intakes from Other Sources – Background
1.4 Identification of Toxicity Reference Values
1.4.1 Classification
1.4.2 Review of Available Values/Information
1.4.3 Recommendation
1.5 Calculated HILs
1.6 References
2 2,4-D
2.1 General
2.2 Previous HIL
2.3 Significance of Exposure Pathways
2.3.1 Oral Bioavailability
2.3.2 Dermal absorption
2.3.3 Inhalation of Dust
2.3.4 Plant Uptake
2.3.5 Intakes from Other Sources – Background
2.4 Identification of Toxicity Reference Values
2.4.1 Classification
2.4.2 Review of Available Values/Information
2.4.3 Recommendation
2.5 Calculated HILs
2.6 References
3 MCPA, MCPB and Mecoprop
3.1 General
3.2 Previous HIL
3.3 Significance of Exposure Pathways
3.3.1 Oral Bioavailability
3.3.2 Dermal absorption
3.3.3 Inhalation of Dust
3.3.4 Plant Uptake
3.3.5 Intakes from Other Sources – Background
3.4 Identification of Toxicity Reference Values
3.4.1 Classification
3.4.2 Review of Available Values/Information
3.4.2.1 MCPA
3.4.2.2 MCPB
3.4.2.3 Mecoprop (MCPP)
3.4.3 Recommendation
3.5 Calculated HILs
3.6 References
4 Picloram
4.1 General
4.2 Previous HIL
4.3 Significance of Exposure Pathways
4.3.1 Oral Bioavailability
4.3.2 Dermal absorption
4.3.3 Inhalation of Dust
4.3.4 Plant Uptake
4.3.5 Intakes from Other Sources – Background
4.4 Identification of Toxicity Reference Values
4.4.1 Classification
4.4.2 Review of Available Values/Information
4.4.3 Recommendation
4.5 Calculated HILs
4.6 References
5 Atrazine
5.1 General
5.2 Previous HIL
5.3 Significance of Exposure Pathways
5.3.1 Oral Bioavailability
5.3.2 Dermal absorption
5.3.3 Inhalation of Dust
5.3.4 Plant Uptake
5.3.5 Intakes from Other Sources – Background
5.4 Identification of Toxicity Reference Values
5.4.1 Classification
5.4.2 Review of Available Values/Information
5.4.3 Recommendation
5.5 Calculated HILs
5.6 References
6 Chlorpyrifos
6.1 General
6.2 Previous HIL
6.3 Significance of Exposure Pathways
6.3.1 Oral Bioavailability
6.3.2 Dermal absorption
6.3.3 Inhalation of Dust
6.3.4 Plant Uptake
6.3.5 Intakes from Other Sources – Background
6.4 Identification of Toxicity Reference Values
6.4.1 Classification
6.4.2 Review of Available Values/Information
6.4.3 Recommendation
6.5 Calculated HILs
6.6 References
7 Bifenthrin
7.1 General
7.2 Previous HIL
7.3 Significance of Exposure Pathways
7.3.1 Oral Bioavailability
7.3.2 Dermal absorption
7.3.3 Inhalation of Dust
7.3.4 Plant Uptake
7.3.5 Intakes from Other Sources – Background
7.4 Identification of Toxicity Reference Values
7.4.1 Classification
7.4.2 Review of Available Values/Information
7.4.3 Recommendation
7.5 Calculated HILs
7.6 References
8 Shortened forms
2,4,5-T is the common name for 2,4,5-trichlorophenoxyacetic acid (or 2,4,5-triphenoxyacetic acid), a chlorophenoxy herbicide.
Several comprehensive reviews of 2,4,5-T in the environment and its toxicity to humans are available and should be consulted for more detailed information not presented in this summary ( OCS 2004; HSDB 2010). The following provides a summary of the key aspects of 2,4,5-T that are relevant to the derivation of a soil HIL.
The herbicide was also commercially produced as an amine salt, alkali metal salt and ester derivative of 2,4,5-T. Pure 2,4,5-T is a white to light tan solid. It is slightly soluble in water whereas the amine and alkali metal salt derivatives are highly soluble. The ester, however, is insoluble in water. 2,3,7,8-tetrachlorodibenzop-dioxin (TCDD), a known human carcinogen, was a common contaminant in the manufacture of 2,4,5-T and its derivatives and was typically present in the low mg/kg to high mg/kg level (OCS 2004). 2,4,5-T with TCDD contamination is now controlled in international trade through the 'Rotterdam Convention’ (Joint FAO/UNEP 2005). It is noted that 2,4,5-T is not expected to persist in the environment for any significant period of time but TCDD will remain and should be considered in a site-specific assessment where a 2,4,5-T source may have been present.
2,4,5-T and its derivatives were introduced in the 1960s and were used as herbicides for broad-leaved wood plants such as blackberries. 2,4,5-T was also combined with the compound 2,4-D to form the ‘agent orange’ herbicide which was widely used by the US military in the Vietnam war (OCS 2004). 2,4,5-T and its derivatives were withdrawn from use in the late 1980s and are no longer approved for use or marketed in Australia.
No previous HIL is available for 2,4,5-T (NEPC 1999).
Insufficient data is available to adequately define the bioavailability of 2,4,5-T, hence a default approach of assuming 100% oral bioavailability has been adopted in the derivation of an HIL. It is noted that a site-specific assessment of bioavailability can be undertaken where required.
Insufficient data is available on the dermal absorption of 2,4,5-T from soil. Hence the default value of 0.1 (10%) suggested by US EPA (1995) for pesticides has been adopted in the derivation of HILs.
2,4,5-T is not considered sufficiently volatile to be of significance and inhalation exposures associated with particulates outdoors and indoors are expected to be of less significance than ingestion of soil. While likely to be negligible, potential inhalation exposures associated with dust have been considered in the HIL derived.
Most chlorophenoxy herbicides are toxic to plants and, as such, will be phytotoxic to almost all broadleaf crops including tomatoes, grapes and fruit trees, well before plant uptake into edible portions of fruit and vegetable crops is of significance. Hence the uptake of these compounds into home-grown produce has not been considered in the derivation of HIL A.
Note that the phytotoxic effects of these compounds may need to be addressed on a site-specific basis if detected in soil.
Review of available publications suggests that very little data is available for Australia. Based on the available information on 2,4,5-T and 2,4-D in the environment, it is likely that background intakes by the general public will be similar to those considered for 2,4-D, which can be considered to be essentially negligible (0%).
The International Agency for Research on Cancer (IARC 1987) has classified chlorophenoxy herbicides as Group 2B—possibly carcinogenic to humans.
US EPA has not classified 2,4,5-T.
Limited data is available on the assessment of carcinogenicity and genotoxicity for 2,4,5-T. Available information on 2,4,5-T is often confounded with the presence of dioxin (TCDD) which was a common contaminant in 2,4,5-T herbicides. 2,4,5-T alone has not been found to be carcinogenic (Joint FAO/UNEP 2005).
On the basis of the available information, it is considered appropriate that a threshold dose-response approach be adopted for 2,4-5-T. The following are available from Level 1 Australian and International sources:
Source | Value | Basis/Comments |
Australian | ||
ADWG (NHMRC 2011) | TDI = 0.03 mg/kg/day | Current drinking water guideline of 0.1 mg/L based on 10% intake from drinking water. Based on equations presented in the ADWG (NHMRC 2011), the TDI considered in this derivation is equal to 0.029 mg/kg/day, essentially equivalent to the ADI available from the Joint FAO/WHO. No further information on the basis for this value is available. |
OCS (2012) | Deleted from current list in 2003. Prior to this, the ADI was listed as 0.03 mg/kg/day. | Previous ADI referenced from Joint FAO/WHO evaluation from 1981. |
International | ||
WHO (1981) | Temporary ADI of 0-0.03 mg/kg/day | Temporary ADI based on a NOEL of 3 mg/kg/day from a rat carcinogenicity study with 2,4,5-T containing 0.05 ppm TCDD. |
WHO (2011) | TDI = 0.003 mg/kg/day | 2,4,5-T has been reviewed in the WHO DWG (originally reviewed and established in 1996,) with a TDI of 0.003 mg/kg/day derived based on a NOAEL for reduced body weight gain, increased liver and kidney weights and renal toxicity in a 2 -year rat study. The same NOAEL was derived for reproductive effects from a three-generation rat study. It is noted that the derivation of the TDI included an additional 10 fold factor to address a suggested association between 2,4,5-T and soft-tissue sarcoma and non-Hodgkin lymphoma (not noted in other reviews available).
2,4,5-T is included in the WHO plan for rolling revisions to the drinking water guidelines. No reviews with respect to this chemical are currently available. |
ATSDR | No evaluation available |
|
US EPA (IRIS 2012) | RfD = 0.01 mg/kg/day
| The US EPA evaluation was established in 1982 and last reviewed in 1988 and provides an oral RfD of 0.01 mg/kg/day, based on a NOAEL of 3 mg/kg/day based on kidney effects in rats, and a 300-fold uncertainty factor.
The value derived is considered protective of reproductive end points. |
The available information from all the above sources is dated. There are some issues with the temporary ADI derived by the Joint FAO/WHO (1981) in that the study considered for the derivation of the ADI included the dioxin (TCDD) contaminant and addressed an end point not associated with 2,4,5-T alone. This value has subsequently been adopted in the derivation of the current ADWG without further review.
The value has been deleted from the current ADI list (OCS 2012). The TDI available in the current WHO DWG (2011) is based on the same studies as considered in 1981, though an additional uncertainty factor has been incorporated to address uncertainties in the database, including potential carcinogenic effects. The basis for this additional factor is not clear, as the carcinogenic effects noted have not been identified in other studies. On this basis, the most appropriate threshold reference value for 2,4,5-T is from US EPA, which is similar to the previous ADI from WHO (and is considered in the current ADWG (NHMRC 2011)).
No dermal or inhalation specific studies or data are available. For the presence of 2,4,5-T in soil, it is considered appropriate to consider use of the available US EPA RfD as a TRV for all pathways of exposures.
On the basis of the discussion above, the following toxicity reference values (TRVs) have been adopted for 2,4,5-T in the derivation of HILs:
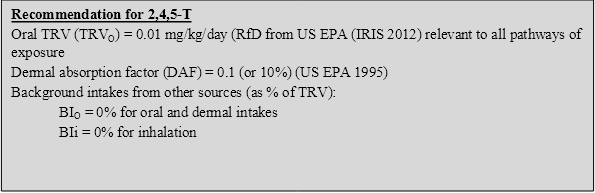
On the basis of the above, the following HILs have been derived for 2,4,5-T (refer to Appendix B for equations used to calculate the HILs and Appendix C for calculations):
HIL Scenario | HIL (mg/kg) | Percentage Contribution from Exposure Pathways | |||
Ingestion of Soil/Dust | Ingestion of Home-grown Produce | Dermal Absorption of Soil/Dust | Inhalation (dust) | ||
Residential A | 600 | 43 | -- | 57 | <1 |
Residential B | 900 | 16 | -- | 84 | <1 |
Recreational C | 800 | 27 | -- | 73 | <1 |
Commercial D | 5000 | 12 | -- | 88 | <1 |
-- Pathway not included in derivation of HIL
HSDB 2010, Hazardous Substances Data Bank, online database available from: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB.
IARC 1987, Summaries and Evaluations, Chlorophenoxy herbicides, Supplement 7: (1987), p.256, International Agency for Research on Cancer.
NEPC 1999, Schedule B (7a), Guideline on Health-Based Investigation Levels, National Environment Protection (Assessment of Site Contamination) Measure, National Environment Protection Council, Australia.
NHMRC 2011, National water quality management strategy, Australian drinking water guidelines, National Health and Medical Research Council, Australia.
OCS 2004, Human Health Risk Assessment of Dioxins in Australia, National Dioxins Program, Technical Report No. 12, Department of the Environment and Heritage, Australian Government, Canberra, Australia.
OCS 2012, ADI List, Acceptable Daily Intakes for Agricultural and Veterinary Chemicals, current to 31 March 2012, Australian Government, Department of Health and Ageing, Office of Chemical Safety (OCS), available from: http://www.health.gov.au/internet/main/publishing.nsf/content/E8F4D2F95D616584CA2573D700770C2A/$File/ADI-apr12.pdf.
Joint FAO/UNEP 2005, Rotterdam Convention on the Prior Informed Consent Procedure for Certain Hazardous Chemicals and Pesticides in International Trade, 2005, Decision Guidance Document: 2,4,5-T and its Salts and Esters, Joint Food and Agriculture Organisation of the United Nations and United Nations Environment Programme for the Operation of the Operation of the Prior Informed Consent, Geneva.
US EPA 1995, Technical Guidance Manual, Assessing Dermal Exposure from Soil, US EPA Region 3, December 1995, available from: http://www.epa.gov/reg3hwmd/risk/human/info/solabsg2.htm.
US EPA (IRIS 2012), data and information available from the Integrated Risk Information System, an online database, available from http://www.epa.gov/iris/.
WHO 1981, Pesticide Residues in Food, Evaluations 1981, FAO Plant Production and Protection Paper 42, Joint FAO/WHO review.
WHO 2011, Guidelines for drinking-water quality, 4th edn, World Health Organization, Geneva, available from http://www.who.int/water_sanitation_health/dwq/chemicals/en/index.html.
2,4-D is the common name for the chlorophenoxy herbicide 2,4-dichlophenoxy acetic acid.
Several comprehensive reviews of 2,4-D in the environment and its toxicity to humans are available and should be consulted for more detailed information not presented in this summary (APVMA 2006; WHO 1984; WHO 1987). The following provides a summary of the key aspects of 2,4-D that are relevant to the derivation of a soil HIL.
The herbicide is also formulated as an amine salt, alkali metal salt and ester derivative of 2,4-D (WHO 1984). Pure 2,4-D is a white to off-white crystalline powder with a slight phenolic odour (APVMA 2006). The commercial grade herbicide is often combined with solvents or surfactants and sold as granules, dust, emulsions and liquid concentrates (WHO 1984). 2,4-D is slightly soluble in water whereas the amine and alkali metal salt derivatives are highly soluble. The ester derivate is insoluble in water (WHO 1984). 2,4-D esters with short chain alcohols are highly volatile whereas 2,4-D and its salt and amine derivatives have a low volatility (APVMA 2006).
Some chlorinated by-products produced during manufacture of 2,4-D and its derivatives such as 2,7- dichlorodibenzo-p-dioxin, 1,3,6,8- and 1,3,7,9-tetrachlorodibenzo-p-dioxins and 1,3,7-trichlorodibenzo-p-dioxin have been associated with enhanced toxicity findings (WHO 1984).
2,4-D and its derivatives are systemic herbicides commonly used in Australia to control broadleaf and aquatic weeds (NHMRC 2004). At least 122 separate products containing these compounds were registered in Australia in 2003 (APVMA 2006). They were registered to control weeds in agricultural crops such as cereals, sugar cane and rice and in pastures and turf. 2,4-D herbicides were also applied at very low application rates to citrus and pears to reduce premature fruit drop and increase fruit storage life (WHO 1984; APVMA 2006). In addition, 2,4-D is used to increase the proportion of medium-sized potato tubers and the intensity of colour in red-skinned varieties (APVMA 2006). In 2006, the Australian Pesticides and Veterinary Medicines Authority conducted a review of the environmental fate and ecotoxicity of volatile 2,4-D esters and concluded that the registration of these compounds should be suspended (APVMA 2006). This review process is ongoing and the APVMA website (www.apvma.gov.au) should be checked for any updates on which products are currently registered.
No previous HIL is available for 2,4-D (NEPC 1999).
Insufficient data is available to adequately define the bioavailability of 2,4-D, hence a default approach of assuming 100% oral bioavailability has been adopted in the derivation of an HIL. It is noted that a site-specific assessment of bioavailability can be undertaken where required.
A dermal absorption value of 0.05 (5%) is available from US EPA (2004) based on a study by Wester et al. (1996). This study evaluated potential dermal absorption of 2,4-D from soil, where absorption over time changed over time (noted to be not-linear). Data from the study showed low absorption over 8 hours (0.03-0.05%) with slightly higher absorption over 16 hours (2.2%). Limited other data is available on the dermal absorption of 2,4-D from soil, hence the value of 0.05 (5%) has been adopted.
2,4-D is not considered sufficiently volatile to be of significance and inhalation exposures associated with particulates outdoors and indoors are expected to be of less significance than ingestion of soil. While likely to be negligible, potential inhalation exposures associated with dust have been considered in the HIL derived.
Most chlorophenoxy herbicides are toxic to plants and, as such, will be phytotoxic to almost all broadleaf crops including tomatoes, grapes and fruit trees well before plant uptake into edible portions of fruit and vegetable crops is of significance. Hence the uptake of these compounds into home-grown produce has not been considered in the derivation of an HIL A.
Note that the phytotoxic effects of these compounds may need to be addressed on a site-specific basis if detected in soil.
Exposure concentrations provided by WHO (1984, 1987) (as well as noted in APVMA (2006)) are derived from areas where 2,4-D is used and is not expected from the presence of 2,4-D contamination in soil. The intakes, however, may be of concern if the HILs were being applied to an area where products containing 2,4-D are used (or have been used in the recent past).
With respect to background intakes of 2,4-D, the following is noted from WHO (1987):
FSANZ (2011) has estimated that the 90th percentile intake of 2,4-D by young children aged 25 years (most sensitive) is 0.014 µg/kg/day or 0.000014 mg/kg/day. This intake is negligible in comparison with the adopted TRV of 0.01 mg/kg/day.
On the basis of the above, background intakes of 2,4-D have been assumed to be essentially negligible (where 2,4-D is not used).
The International Agency for Research on Cancer (IARC 1987) has classified chlorophenoxy herbicides as Group 2B—possibly carcinogenic to humans.
US EPA has not classified 2,4-D.
There is limited information on the assessment of carcinogenicity and genotoxicity for 2,4-D from IARC and US EPA. Ibrahim et al. (1991) provided a summary of a review of carcinogenicity of 2,4-D following review by a panel of 13 scientists. Based on a weight-of-evidence approach 2,4-D was considered unlikely to be a genotoxic carcinogen because it has not been shown to be mutagenic in most in vitro and in vivo systems. The predominant opinion from the panel was that the weight of evidence indicates that it is possible that exposure to 2,4-D may cause cancer in humans.
On the basis of the available information, it is considered appropriate that a threshold dose-response approach be adopted for 2,4-D. The following are available from Level 1 Australian and International sources:
Source | Value | Basis/Comments |
Australian | ||
ADWG (NHMRC 2011) | TDI = 0.01 mg/kg/day | Current ADWG (NHMRC 2011) of 0.03 mg/L based on 10% intake from drinking water. Based on equations presented in the ADWG, the TDI considered in this derivation is equal to 0.009 mg/kg/day, which can be rounded to 0.01 mg/kg/day, essentially equivalent to the ADI available from the OCS. |
OCS (2012) | ADI = 0.01 mg/kg/day | The ADI is noted to have been last reviewed in June 2006 and is based on a NOEL of 1 mg/kg/day associated with abnormal renal morphology in a 2-year rat study, supported by the same NOELs (based on kidney effects) in a 2-year mouse and 1-year dog study. |
International | ||
WHO (2011) | ADI = 0.01 mg/kg/day | ADI, used in the derivation of the current WHO DWG (2011), was established by JMPR (FAO/WHO 1997) for 2,4-D and its salts and esters on the basis of a NOAEL of 1 mg/kg/day in a 1-year toxicity study in dogs and 2-year study in rats, and an uncertainty factor of 100-fold. |
ATSDR | No evaluation available |
|
US EPA (IRIS 2012) | RfD = 0.01 mg/kg/day
| US EPA has derived an oral RfD of 0.01 mg/kg/day. The value was last reviewed in 1986 and is derived based on a LOAEL of 1 mg/kg/day associated with abnormal renal morphology from a 90-day rat bioassay and a 1-year interim report from a 2 year rat study, and an uncertainty factor of 100. |
Based on the available data above, there is general agreement from Australian and international sources on the consideration of an oral toxicity reference value of 0.01 mg/kg/day.
No dermal or inhalation specific studies or data are available. For the presence of 2,4-D in soil (not during use), it is considered appropriate to consider the use of the available ADI for all pathways of exposures.
On the basis of the discussion above, the following toxicity reference values (TRVs) have been adopted for 2,4-D in the derivation of HILs:

On the basis of the above, the following HILs have been derived for 2,4-D (refer to Appendix B for equations used to calculate the HILs and Appendix C for calculations):
HIL Scenario | HIL (mg/kg) | Percentage Contribution from Exposure Pathways | |||
Ingestion of Soil/Dust | Ingestion of Home-grown Produce | Dermal Absorption of Soil/Dust | Inhalation (dust) | ||
Residential A | 900 | 59 | -- | 41 | <1 |
Residential B | 1600 | 27 | -- | 73 | <1 |
Recreational C | 1300 | 43 | -- | 57 | <1 |
Commercial D | 9000 | 21 | -- | 79 | <1 |
-- Pathway not included in derivation of HIL
APVMA 2006, Preliminary Review Finding (Environment) Part 1: 2,4-D Esters. The Reconsideration of Approvals of the Active Constituents 2,4-D, Registrations of Products Containing 2,4-D and their Associated Labels, Australian Pesticides and Veterinary Medicines Authority, Canberra, Australia.
IARC 1987, Summaries and Evaluations, Chlorophenoxy herbicides, Supplement 7: (1987), p.256, International Agency for Research on Cancer.
Ibrahim ,MA, Bond, GG, Burke, TA, Cole, P, Dost, FN, Enterline, PE, Gough, M, Greenberg, RS, Halperin, WE, McConnell, E, Munro, IC, Swenberg, JA, Zahm, SH & Graham, JD 1991, ‘Weight of the Evidence on the Human Carcinogenicity of 2,4-D’, Environmental Health Perspectives, vol. 96, pp. 213222.
FAO/WHO (1997), Pesticide residues in food — 1996, Evaluations 1996, Part II — Toxicological, World Health Organization, International Programme on Chemical Safety, Joint FAO/WHO Meeting on Pesticide Residues (WHO/PCS/97.1), Geneva.
FSANZ 2011, The 23rd Australian Total Diet Study, Food Standards Australia and New Zealand.
NEPC 1999, Schedule B (7a), Guideline on Health-Based Investigation Levels, National Environment Protection (Assessment of Site Contamination) Measure, National Environment Protection Council, Australia.
NHMRC 2011, National water quality management strategy, Australian drinking water guidelines, National Health and Medical Research Council, Australia.
OCS 2012, ADI List, Acceptable Daily Intakes for Agricultural and Veterinary Chemicals, current to 31 March 2012, Australian Government, Department of Health and Ageing, Office of Chemical Safety (OCS), available from: http://www.health.gov.au/internet/main/publishing.nsf/content/E8F4D2F95D616584CA2573D700770C2A/$File/ADI-apr12.pdf.
US EPA 2004, Risk Assessment Guidance for Superfund, Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment), Final, EPA/540/R/99/005, OSWER 9285.7-02EP, July 2004.
US EPA (IRIS 2012), data and information available from the Integrated Risk Information System, an online database, available from http://www.epa.gov/iris/.
Wester, RC, Melendres, J, Logan, F, Hui, X, & Maibach, HI 1996, ‘Percutaneous Absorption of 2,4-Dichlorophenoxyacetic Acid from Soil with Respect to the Soil Load and Skin Contact Time: In-vivo Absorption in Rhesus Monkey and in Vitro Absorption in Human Skin’, J. Toxicol. Environ. Health, vol. 47, pp. 335344.
WHO 1984, Environmental Health Criteria No 29 2,4- Dichlorophenoxy Acetic Acid (2,4-D), World Health Organization, Geneva.
WHO 1987, Health and Safety Guide No. 5, 2,4-Dichlorophenoxyacetic (2,4-D), IPCS International Programme on Chemical Safety.
WHO 2011, Guidelines for drinking-water quality, 4th edn, World Health Organization, Geneva, available from http://www.who.int/water_sanitation_health/dwq/chemicals/en/index.html.
The following information on MCPA (4-chloro-2-methylphenoxyacetic acid), MCPB (4-(2-methyl-4-chlorophenoxy)butyric acid) and mecoprop (also referenced as MCPP) are grouped together as they are structurally similar chlorophenoxy herbicides.
While limited data is available, reviews of these compounds in the environment and their toxicity to humans are available and should be consulted for more detailed information not presented in this summary (WHO 2011; HSDB 2010). The following provides a summary of the key aspects of these compounds that are relevant to the derivation of a soil HIL.
In their pure form the three compounds are white crystalline solids, though technical grade products can be white to light brown crystal powders or liquids. The compounds are often formulated as salts (e.g. potassium or diethylamine salts) or esters (e.g. iso-octyl esters). The three compounds are the active ingredients in post emergence herbicides used to control annual and perennial weeds in agricultural, commercial/industrial and domestic environments. In Australia all three compounds are registered for agricultural application on wheat, barley, oats, sorghum, rice, linseed, peas, grass pastures, turf, clover, corn/maize and oilseed poppies, and for the home garden to control broadleaf weeds (WHO 2011).
No previous HIL is available for MCPA, MCPB or mecoprop (NEPC 1999).
Insufficient data is available to adequately define the bioavailability of MCPA, MCPB or mecoprop, hence a default approach of assuming 100% oral bioavailability has been adopted in the derivation of an HIL. It is noted that a site-specific assessment of bioavailability can be undertaken where required.
Insufficient data is available on the dermal absorption of MCPA, MCPB or mecoprop from soil. Hence the default value of 0.1 (10%) suggested by US EPA (1995) for pesticides has been adopted in the derivation of HILs.
MCPA, MCPB and mecoprop are not considered sufficiently volatile to be of significance and inhalation exposures associated with particulates outdoors and indoors are expected to be of less significance than ingestion of soil. While likely to be negligible, potential inhalation exposures associated with dust have been considered in the HIL derived.
Most chlorophenoxy herbicides are toxic to plants and, as such, will be phytotoxic to almost all broadleaf crops including tomatoes, grapes and fruit trees well before plant uptake into edible portions of fruit and vegetable crops is of significance. Hence the uptake of these compounds into home-grown produce has not been considered in the derivation of HIL A.
Note that the phytotoxic effects of these compounds may need to be addressed on a site-specific basis if detected in soil.
Limited data is available for the assessment of background intakes of MCPA, MCPB and mecoprop. These compounds are currently registered for use in Australia (while some areas are only allowed controlled use of MCPA) and they are generally not considered persistent in the environment. The compounds are not included in the Australian Total Diet Surveys (FSANZ 2003; FSANZ 2011) and there is no data regarding concentrations in drinking water or air in Australia. Away from areas where these herbicides are used, exposure by the general public is expected to be low. In the USA, MCPA was detected up to 0.54 µg/L in surface waters and up to 5.5 µg/L in groundwater (WHO 2011). Background intakes may be similar to those considered for 2,4-D, which is essentially negligible (where these products are not used).
The International Agency for Research on Cancer (IARC 1987) has classified chlorophenoxy herbicides as Group 2B—possibly carcinogenic to humans. Information provided in the IARC evaluation relates more specifically to MCPA and mecoprop. No evaluation is available for MCPB.
US EPA has not classified MCPA, MCPB or mecoprop.
There is limited information on the assessment of carcinogenicity and genotoxicity for these compounds. WHO (2011) notes that recent studies on rats and mice do not indicate that MCPA was carcinogenic and there is only limited and inconclusive data on the genotoxicity of MCPA. Limited studies available on MCPB and mecoprop were negative with respect to genotoxicity. On the basis of the available information, it is considered appropriate that a threshold doseresponse approach be adopted for these herbicides. The following are available from Level 1 Australian and International sources:
Source | Value | Basis/Comments |
Australian | ||
ADWG (NHMRC 2011) | TDI = 0.011 mg/kg/day | MCPA has been assessed with a health-based guideline of 0.04 mg/L based on a TDI of 0.011 mg/kg/day based on a NOEL of 1.1 mg/kg/day from a 2-year study in rats, and an uncertainty factor of 100 |
OCS (2012) | ADI = 0.01 mg/kg/day | The ADI is noted to have been set in April 1994 and is based on a NOEL of 1.1 mg/kg/day (as considered in the ADWG (NHMRC 2011)). |
International | ||
WHO (2011) | TDI = 0.0005 mg/kg/day | The TDI was derived on the basis of a NOAEL of 0.15 mg/kg/day associated with renal and liver toxicity observed in a 1-year feeding study in dogs, and an uncertainty factor of 300. It is noted that the current guideline has remained unchanged since first derived in 1993. MCPA is included in the rolling revisions to the WHO DWG (2011) with no significant revisions issued to date. |
ATSDR | No evaluation available |
|
US EPA (IRIS 2012) | RfD = 0.0005 mg/kg/day
| The RfD (last reviewed in 1987) is derived based on the same study and evaluation provided in the WHO DWG (2011). |
Source | Value | Basis/Comments |
Australian | ||
ADWG (NHMRC 2011) | No evaluation available |
|
OCS (2012) | ADI = 0.01 mg/kg/day | The ADI is noted to have been set in May 1994 and is based on a NOEL of 1.1 mg/kg/day. |
International | ||
WHO (2011) | No quantitative value available | Insufficient data was available to establish a guideline value for MCPB in drinking water. |
ATSDR | No evaluation available |
|
US EPA (IRIS 2012) | RfD = 0.01 mg/kg/day
| The RfD (last reviewed in 1991) is derived based on a NOEL of 12 mg/kg/day associated with reproductive effects in a 13-week feeding study with dogs, and an uncertainty factor of 1000. |
Source | Value | Basis/Comments |
Australian | ||
ADWG (NHMRC 2011) | No evaluation available |
|
OCS (2012) | ADI = 0.01 mg/kg/day | The ADI is noted to have been set in July 1998 and is based on a NOEL of 1 mg/kg/day, and an uncertainty factor of 100. |
International | ||
WHO (2011) | TDI = 0.0033 mg/kg/day | The TDI was derived on the basis of a NOAEL of 1 mg/kg/day associated with kidney effects in 1- and 2-year studies in rats, and an uncertainty factor of 300. It is noted that the current guideline has remained unchanged since first published in 1996. Mecoprop is included in the rolling revisions to the WHO DWG (2011) with no significant revisions issued to date. |
ATSDR | No evaluation available |
|
US EPA (IRIS 2012) | RfD = 0.001 mg/kg/day
| The RfD (last reviewed in 1990) is derived based on a NOEL of 3 mg/kg/day associated with kidney effects in a 90-day rat feeding study, and an uncertainty factor of 3000. |
The available evaluations in relation to MCPA, MCPB and mecoprop are all dated (none more recent than 1996) and are based on limited databases of studies. In relation to MCPB, the evaluations available from OCS (2012) and US EPA are consistent. In relation to MCPA and mecoprop, the critical studies identified for the determination of the point of departure differ between the OCS and WHO/US EPA evaluations. The subsequent application of uncertainty factors (with WHO/US EPA more conservative) also differs. Insufficient data is available to support any one evaluation, hence preference has been given to the Australian values adopted by OCS (2012), which have also been adopted in the derivation of the Australian Drinking Water Guidelines (NHMRC 2011). On this basis, the current Australian ADIs (as presented by OCS (2012)) have been adopted for the derivation of soil HILs.
No dermal or inhalation-specific studies or data are available. For the presence of MCPA, MCPB and mecoprop in soil (not during use) it is considered appropriate to consider use of the available ADI for all pathways of exposures.
On the basis of the discussion above, the following toxicity reference values (TRVs) have been adopted for MCPA, MCPB and mecoprop in the derivation of HILs:
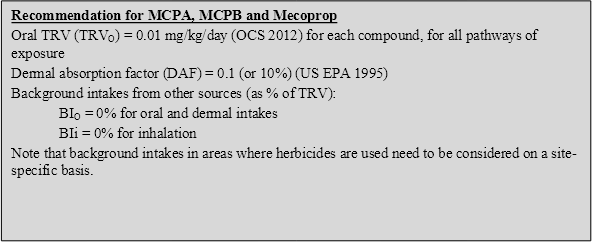
On the basis of the above, the following HILs have been derived for MCPA, MCPB and mecoprop (as individual compounds) (refer to Appendix B for equations used to calculate the HILs and Appendix C for calculations):
HIL Scenario | HIL (mg/kg) | Percentage Contribution from Exposure Pathways | |||
Ingestion of Soil/Dust | Ingestion of Home-grown Produce | Dermal Absorption of Soil/Dust | Inhalation (dust) | ||
Residential A | 600 | 43 | -- | 57 | <1 |
Residential B | 900 | 16 | -- | 84 | <1 |
Recreational C | 800 | 27 | -- | 73 | <1 |
Commercial D | 5000 | 12 | -- | 88 | <1 |
-- Pathway not included in derivation of HIL
FSANZ 2003, The 20th Australian Total Diet Survey, a total diet survey of pesticide residues and contaminants, website: http://www.anzfa.gov.au/.
FSANZ 2011, The 23rd Australian Total Diet Study, Food Standards Australia and New Zealand.
HSDB (2010), Hazardous Substances Data Bank, online database available from: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB.
IARC 1987, Summaries and Evaluations, Chlorophenoxy herbicides, Supplement 7, (1987), p.256, International Agency for Research on Cancer.
NEPC 1999, Schedule B (7a), Guideline on Health-Based Investigation Levels, National Environment Protection (Assessment of Site Contamination) Measure, National Environment Protection Council, Australia.
NHMRC 2011, National water quality management strategy, Australian drinking water guidelines, National Health and Medical Research Council, Australia.
OCS 2012, ADI List, Acceptable Daily Intakes for Agricultural and Veterinary Chemicals, current to 31 March 2012, Australian Government, Department of Health and Ageing, Office of Chemical Safety (OCS), available from: http://www.health.gov.au/internet/main/publishing.nsf/content/E8F4D2F95D616584CA2573D700770C2A/$File/ADI-apr12.pdf.
US EPA 1995, Technical Guidance Manual, Assessing Dermal Exposure from Soil, US EPA Region 3, December 1995, available from: http://www.epa.gov/reg3hwmd/risk/human/info/solabsg2.htm.
US EPA (IRIS 2012), data and information available from the Integrated Risk Information System, an online database, available from http://www.epa.gov/iris/.
WHO 2011, Guidelines for drinking-water quality, 4th edition, World Health Organization, Geneva, available from http://www.who.int/water_sanitation_health/dwq/chemicals/en/index.html
Limited data is available on picloram, however reviews of this compound in the environment and its toxicity to humans are available and should be consulted for more detailed information not presented in this summary (Health Canada 1988; US EPA 1995a; OEHHA 1997). The following provides a summary of the key aspects of picloram that are relevant to the derivation of a soil HIL.
Picloram is a member of the pyridine carboxylic acid group and is manufactured in a number of forms. Picloram acid is only manufactured as an intermediate product in the production of herbicides whereas the amine salt, potassium salt and ester derivatives of picloram are produced as commercial herbicides. Technical grade picloram acid is an off-white to brown powder. It is slightly soluble in water and the amine and potassium salt derivatives are highly soluble. The ester derivative, however, is insoluble in water (US EPA 1995a).
Picloram acid and its derivatives have been used since the 1960s as a systemic herbicide to control woody plants and broadleaf weeds in rights of way, forestry, rangeland and pasture. In Australia, picloram derivatives are used to control weeds in winter cereals and linseed crops and to control a number of environmental and noxious weeds (APVMA 2009).
Picloram products are commonly contaminated with hexachlorobenzene (HCB). The presence of HCB in picloram affects the assessment of toxicity in a number of studies. Limited data is available for picloram alone. Available data also show that picloram is synergistic with several common herbicides (in particular 2,4-D, atrazine and alachlor) with respect to its toxicity to mammals and fish (NCAP 1998).
No previous HIL is available for picloram (NEPC 1999).
Insufficient data is available to adequately define the bioavailability of picloram, hence a default approach of assuming 100% oral bioavailability has been adopted in the derivation of an HIL. It is noted that a site-specific assessment of bioavailability can be undertaken where required.
Insufficient data is available on the dermal absorption of picloram from soil. Hence the default value of 0.1 (10%) suggested by US EPA (1995b) for pesticides has been adopted in the derivation of HILs.
Picloram is not considered sufficiently volatile to be of significance and inhalation exposures associated with particulates outdoors and indoors are expected to be of less significance than ingestion of soil. While likely to be negligible, potential inhalation exposures associated with dust have been considered in the HIL derived.
Most carboxylic herbicides are toxic to plants and, as such, will be phytotoxic to almost all broadleaf crops including tomatoes, grapes and fruit trees well before plant uptake into edible portions of fruit and vegetable crops is of significance. Hence the uptake of these compounds into home-grown produce has not been considered in the derivation of HIL A.
Note that the phytotoxic effects of these compounds may need to be addressed on a site-specific basis if detected in soil.
Limited data is available for the assessment of background intakes of picloram. Picloram products are currently registered for use in Australia and the compound is considered persistent in the environment. Picloram is not included in the Australian Total Diet Surveys (FSANZ 2003; FSANZ 2011) and there is no data regarding concentrations in drinking water or air in Australia. Away from areas where picloram products are used, exposure by the general public is expected to be low. Review by US EPA (1995b) suggests that dietary intakes comprise only 0.5% of the threshold reference value (RfD) adopted (0.2 mg/kg/day) for most of the US population, with intakes from non-nursing infants highest at 1.9% of the RfD adopted. Review by Health Canada (1988) also noted the maximum dietary intake of picloram is estimated to be negligible, based on available data in Canada and the USA. On this basis, intakes from other sources have been assumed to be negligible in the derivation of HILs.
The International Agency for Research on Cancer (IARC 1991) has classified picloram as Group 3—not classifiable.
US EPA has not classified picloram.
Studies associated with the assessment of carcinogenicity of picloram are noted to be affected by the presence of HCB as a contaminant/impurity. Hence a number of reviews of carcinogenicity are conflicting. The review by IARC noted limited evidence of carcinogenicity for technical grade picloram in experimental animals. In general, the available data suggests the picloram is not genotoxic (Health Canada 1988; US EPA 1995) or at most weakly mutagenic (OEHHA 1997). On the basis of the limited available information, it is considered appropriate that a threshold doseresponse approach be adopted for picloram. The following are available from Level 1 Australian and International sources:
Source | Value | Basis/Comments |
Australian | ||
ADWG (NHMRC 2011) | TDI = 0.07 mg/kg/day | The current ADWG (NHMRC 2011) derive a guideline of 0.3 mg/L derived from a NOEL of 7 mg/kg/day associated with increased liver weights in a short-term dietary study in rats, and an uncertainty factor of 100. |
OCS (2012) | ADI = 0.07 mg/kg/day | The ADI is noted to have been set in February 1987 and is based on a NOEL of 7 mg/kg/day (as considered in the ADWG, noted above). |
International | ||
WHO(2011) | No evaluation available |
|
ATSDR | No evaluation available |
|
Health Canada (1988) | NDI = 0.02 mg/kg/day | Negligible daily intake (NDI) derived on the basis of a NOAEL of 20 mg/kg/day associated with liver and kidney changes in rat and mouse studies, and an uncertainty factor of 1000. |
US EPA (IRIS 2012) | RfD = 0.07 mg/kg/day
| The RfD (last reviewed in 1987) is derived based on the same study and evaluation provided in the ADWG (NHMRC 2004). Value also derived by OEHHA (1997). |
US EPA (1995) | RfD = 0.2 mg/kg/day | RfD calculated based on a NOEL of 20 mg/kg/day from a 2-year chronic rat feeding study, and an uncertainty factor of 100. |
Limited quantitative data is available for picloram, however it is recommended that the current Australian ADI/TDI be adopted for the derivation of a soil HIL.
No dermal or inhalation-specific studies or data are available. For the presence of picloram in soil (not during use), it is considered appropriate to consider use of the available ADI for all pathways of exposures.
On the basis of the discussion above, the following toxicity reference values (TRVs) have been adopted for picloram in the derivation of HILs:

On the basis of the above, the following HILs have been derived for picloram (refer to Appendix B for equations used to calculate the HILs and Appendix C for calculations):
HIL Scenario | HIL (mg/kg) | Percentage Contribution from Exposure Pathways | |||
Ingestion of Soil/Dust | Ingestion of Home-grown Produce | Dermal Absorption of Soil/Dust | Inhalation (dust) | ||
Residential A | 4500 | 43 | -- | 57 | <1 |
Residential B | 6600 | 16 | -- | 84 | <1 |
Recreational C | 5700 | 27 | -- | 73 | <1 |
Commercial D | 35 000 | 12 | -- | 88 | <1 |
-- Pathway not included in derivation of HIL
APVMA 2009, Chemicals Nominated for Review, last update unknown, accessed July 2009, Australian Pesticides and Veterinary Medicines Authority (APVMA), http://www.apvma.gov.au/chemrev/ChemRevProgram.shtml.
FSANZ 2003, The 20th Australian Total Diet Survey, a total diet survey of pesticide residues and contaminants. website: http://www.anzfa.gov.au/.
FSANZ 2011, The 23rd Australian Total Diet Study, Food Standards Australia and New Zealand.
Health Canada 1988, Picloram, Environmental and Workplace Health, reviewed in 1990, available from: http://www.hc-sc.gc.ca/ewh-semt/pubs/water-eau/picloram-piclorame/index-eng.php.
IARC 1991, Summaries and Evaluations, Picloram, vol. 53 (1991), p,481, International Agency for Research on Cancer.
NEPC 1999, Schedule B (7a), Guideline on Health-Based Investigation Levels, National Environment Protection (Assessment of Site Contamination) Measure, National Environment Protection Council, Australia.
NCAP 1998, ‘Picloram, Herbicide Fact Sheet’, Northwest Coalition for Alternatives to Pesticides, Journal of Pesticide Reform, Spring 1998, vol. 18, No.1.
NHMRC 2011, National water quality management strategy, Australian drinking water guidelines, National Health and Medical Research Council, Australia.
OCS 2012, ADI List, Acceptable Daily Intakes for Agricultural and Veterinary Chemicals, current to 31 March 2012, Australian Government, Department of Health and Ageing, Office of Chemical Safety (OCS), available from: http://www.health.gov.au/internet/main/publishing.nsf/content/E8F4D2F95D616584CA2573D700770C2A/$File/ADI-apr12.pdf.
OEHHA 1997, Public Health Goal for Picloram in Drinking Water, prepared by Pesticide and Environmental Toxicology Section, Office of Environmental Health Hazard Assessment, California Environmental Protection Agency, December 1997.
US EPA (IRIS 2012), data and information available from the Integrated Risk Information System, an online database, available from http://www.epa.gov/iris/.
US EPA 1995a, Reregistration Eligibility Decision (RED) Office of Prevention, Pesticides and Toxic Substances, United States Environment Protection Agency, Washington, DC.
US EPA 1995b, Technical Guidance Manual, Assessing Dermal Exposure from Soil, US EPA Region 3, December 1995, available from: http://www.epa.gov/reg3hwmd/risk/human/info/solabsg2.htm.
WHO 2011, Guidelines for drinking-water quality, 4th edn, World Health Organization, Geneva, available from http://www.who.int/water_sanitation_health/dwq/chemicals/en/index.html.
Several comprehensive reviews of atrazine in the environment and its toxicity to humans are available and should be consulted for more detailed information not presented in this summary (ATSDR 2003; NRA 1997; APVMA 2008; IARC 1999). The following provides a summary of the key aspects of atrazine that are relevant to the derivation of a soil HIL.
Atrazine is the common name for the compound 6-chloro-N2-ethyl-N4-isopropyl-1,3,5-triazine-2,4-diamine which is an odourless white powder or colourless crystal (ATSDR 2003). Commercially manufactured atrazine is typically greater that 90% pure. Common impurities include dichlorotriazines, hydroxytriazines, tris(alkyl)aminotriazines, simazine, propazine and sodium chloride (ATSDR 2003). Atrazine is manufactured as a liquid, granules or wettable powder and can also be formulated in combination with other herbicides such as ametryn, amitrole, hexazinone, metalochlor, glyphosate and dicamba (NRA 1997).
Atrazine is one of the most widely used herbicides in Australian agriculture and has been used since the 1960s (NRA 1997). It is primarily used to control broadleaf weeds and some grasses between crops such as sorghum, maize, lupins, sugar cane and triazine-tolerant canola. Atrazine is also widely used to control weeds and some grasses by the forestry industry in pine and eucalyptus plantations (NRA 1997; NHMRC 2011). Non-agricultural uses in Australia such as the spraying of weeds along fence lines, irrigation channels, drains, driveways and footpaths were discontinued in 1995 (NRA 1997).
Regulatory actions (by the National Registration Authority for Agricultural and Veterinary Chemicals [APVMA]) undertaken in 1997 included cancellation of industrial and non-agricultural uses of atrazine (home garden uses and all commercial turf uses), deletion of use patterns and maximum residue limits (MRLs) for label claims for which there were no current use patterns (citrus, grapes and pineapples) and the introduction of a range of label instructions to reduce the risk of atrazine entering waterways. In addition, registrants were required to provide additional residue and monitoring data.
The APVMA has initiated a project to re-examine the possibility that the triazines (atrazine and related chemicals with a similar MoA) may have unintended harmful effects on humans, taking into account ongoing research into a newly hypothesised endocrine MoA. This project will take into account international reports, such as the work of the Joint Meeting on Pesticide Residues (JMPR).
Registrants who have a product whose label specifies a claim for weed control on triazine-tolerant canola will be required to either generate additional data or include an additional label restraint that specifies that atrazine must not be used post-emergence on triazine-tolerant canola grown on raised beds.
After consideration of the additional assessments completed after 1997, APVMA accepts the recommendations of OCS and the 2004 recommendations of DEWHA, and the following regulatory actions have been applied:
These variations to label instructions satisfy the requirements for continued registration of products; and so
As an associated outcome of the review, changes will be made to the MRL Standard to align entries in the standard with existing approved use patterns.
No previous HIL is available for atrazine (NEPC 1999).
Insufficient data is available to adequately define the bioavailability of atrazine hence a default approach of assuming 100% oral bioavailability has been adopted in the derivation of an HIL. It is noted that a site-specific assessment of bioavailability can be undertaken where required.
Insufficient data is available on the dermal absorption of atrazine from soil. Hence the default value of 0.1 (10%) suggested by US EPA (1995) for pesticides has been adopted in the derivation of HILs.
Atrazine is not considered sufficiently volatile to be of significance and inhalation exposures associated with particulates outdoors and indoors are expected to be of less significance than ingestion of soil. While likely to be negligible, potential inhalation exposures associated with dust have been considered in the HIL derived.
Atrazine is used as a herbicide and, as such, is phytotoxic to almost all broadleaf weeds and plants. Some plants are more sensitive than others to residues of atrazine in the soil, however in general, phytotoxicity will occur well before plant uptake into edible portions of fruit and vegetable crops is of significance. Hence the uptake of these compounds into home-grown produce has not been considered in the derivation of an HIL A.
Note that the persistence of atrazine in soil and potential for phytotoxic effects may need to be addressed on a site-specific basis if detected in soil.
Reviews of potential intakes from sources other than soil (primarily food) by NRA (1997), NHMRC (2011) and RIVM (2001) suggested these intakes were essentially negligible. Further review of residue data by APVMA (2008) noted that, when atrazine was used in accordance with the revised label directions, residues were unlikely to pose a risk to human health. Potential exposures during application of atrazine products may require further consideration on a site-specific basis; however exposures by the general public (in areas away from application) are negligible.
The International Agency for Research on Cancer (IARC 1999) has classified atrazine as Group 3—not classifiable. US EPA has not classified atrazine.
The available data reviewed by JMPR (2007) and APVMA (2008) suggested that atrazine was not likely to pose a carcinogenic risk to humans. Review by JMPR (2007) and RIVM (2001) suggested that based on the weight of evidence, atrazine was not genotoxic. There is some evidence that it can induce mammary tumours in rats as a result of hormonal changes, but the mechanism is believed to be non-genotoxic. On the basis of the available information, it is considered appropriate that a threshold doseresponse approach be adopted for atrazine.
The following are available from Level 1 Australian and International sources:
Source | Value | Basis/Comments |
Australian | ||
ADWG (NHMRC 2011) | ADI = 0.005 mg/kg/day | Current ADWG (NHMRC 11) of 0.04 mg/L based on 50% intake from drinking water and an ADI of 0.005 mg/kg/day as referenced from the TGA (NRA 1997). |
OCS (2012) | ADI = 0.005 mg/kg/day | The ADI of 0.005 mg/kg/day is noted to be based on a NOEL of 10ppm associated with mammary tumours from a 24-month female rat study, and a 100-fold safety factor. This value was set in December 1996. |
NRA (1997) | ADI = 0.005 mg/kg/day | The NRA (1997) review identified the relevance of adopting an ADI of 0.005 mg/kg/day for atrazine. This value has been reconfirmed in the update provided by APVMA (2008). However the review noted that APVMA has initiated a project to re-examine the possibility that the triazines may have harmful endocrine effects, including updates available from JMPR. APVMA also note that US EPA is currently reviewing atrazine. |
International | ||
JMPR (2007) | ADI = 0.02 mg/kg/day | Review of atrazines by the Joint FAO/WHO Meeting on Pesticides Residues (JPMR, 2007) identified a group ADI (for atrazine, diethyl-atrazine, di-isopropyl-atrazine and diaminochlorotriazine) of 00.02 mg/kg/day based on oestrous cycle disruption. |
WHO (2011) | ADI = 0.02 mg/kg/day | Group ADI for atrazine and its chloro-s-triazine metabolites (reviewed in 2011) is based on a NOAEL of 1.8 mg/kg/day identified on the basis of luteinizing hormone surge suppression and subsequent disruption of the oestrous cycle seen at 3.6 mg/kg body weight per day in a 6-month study in rats, using a safety factor of 100 |
RIVM (2001) | TDI = 0.005 mg/kg/day | TDI based on a NOAEL of 0.5 mg/kg/day associated with reproductive effects in rats, and a 100-fold uncertainty factor. |
ATSDR | No evaluation available |
|
US EPA (IRIS 2012) | RfD = 0.035 mg/kg/day
| The US EPA (available from IRIS) have derived an oral RfD of 0.035 mg/kg/day. The value was last reviewed in 1993 and is based on a NOAEL of 3.5 mg/kg/day associated with decreased body weight gain from a 2-year rat study, and an uncertainty factor of 100. |
While the most recent review by WHO (2011) provides a less conservative ADI, the current Australian ADI of 0.005 mg/kg/day is considered relevant and appropriate for consideration in the derivation of a soil HIL.
No dermal or inhalation-specific studies or data are available. For the presence of atrazine in soil (not during use in herbicide products), it is considered appropriate to consider use of the available threshold ADI for all pathways of exposures.
On the basis of the discussion above the following toxicity reference values (TRVs) have been adopted for atrazine in the derivation of HILs:

On the basis of the above, the following HILs have been derived for atrazine (refer to Appendix B for equations used to calculate the HILs and Appendix C for calculations):
HIL Scenario | HIL (mg/kg) | Percentage Contribution from Exposure Pathways | |||
Ingestion of Soil/Dust | Ingestion of Home-grown Produce | Dermal Absorption of Soil/Dust | Inhalation (dust) | ||
Residential A | 320 | 43 | -- | 57 | <1 |
Residential B | 470 | 16 | -- | 84 | <1 |
Recreational C | 400 | 27 | -- | 73 | <1 |
Commercial D | 2500 | 12 | -- | 88 | <1 |
-- Pathway not included in derivation of HIL
APVMA 2008, Atrazine, Final Review Report and Regulatory Decision, Australian Pesticides & Veterinary Medicines Authority, March 2008.
ATSDR 2003, Toxicological Profile for Atrazine, US Department of Health and Human Services, ATSDR, September 2003, available from http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=338&tid=59.
IARC 1999, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Some Chemicals That Cause Tumors of the Lungs or Urinary Bladder in Rodents and Some Other Substances, World Health Organization, International Agency for Research on Cancer, Lyon, France.
JMPR 2007, Pesticide Residues in Food, 2007, Joint FAO/WHO Meeting on Pesticide Residues, FAO Plant Production Paper 191, 2007.
NEPC 1999, Schedule B (7a), Guideline on Health-Based Investigation Levels, National Environment Protection (Assessment of Site Contamination) Measure, National Environment Protection Council, Australia.
NHMRC 2011, National water quality management strategy, Australian drinking water guidelines, National Health and Medical Research Council, Australia.
NRA 1997, Review Summary on the NRA Review of Atrazine, Existing Chemicals Review Program, National Registration Authority for Agricultural and Veterinary Chemicals 1997, National Registration Authority for Agricultural and Veterinary Chemicals, Commonwealth of Australia, Canberra, Australia.
OCS 2012, ADI List, Acceptable Daily Intakes for Agricultural and Veterinary Chemicals, current to 31 March 2012, Australian Government, Department of Health and Ageing, Office of Chemical Safety (OCS), available from: http://www.health.gov.au/internet/main/publishing.nsf/content/E8F4D2F95D616584CA2573D700770C2A/$File/ADI-apr12.pdf.
RIVM 2001, Re-evaluation of human-toxicological Maximum Permissible Risk levels, National Institute of Public Health and the Environment, Bilthoven, Netherlands, available from: http://www.rivm.nl/bibliotheek/rapporten/711701025.html.
US EPA 1995, Technical Guidance Manual, Assessing Dermal Exposure from Soil, US EPA Region 3, December 1995, available from: http://www.epa.gov/reg3hwmd/risk/human/info/solabsg2.htm.
US EPA (IRIS 2012) data and information available from the Integrated Risk Information System, an online database, available from http://www.epa.gov/iris/.
WHO 2011, Guidelines for drinking-water quality, 4th edn, World Health Organization, Geneva, available from http://www.who.int/water_sanitation_health/dwq/chemicals/en/index.html.
Several reviews of chlorpyrifos in the environment and its toxicity to humans are available and should be consulted for more detailed information not presented in this summary (ATSDR 1997; WHO 2004; NRAAVC 2000; APVMA 2009; Taylor & Di Marco 2003). The following provides a summary of the key aspects of chlorpyrifos that are relevant to the derivation of a soil HIL.
Chlorpyrifos is the common name for the organophosphorous insecticide O,O-diethyl O-3,5,6-trichloro-2-pyridyl phophorothioate. Pure chlorpyrifos is an odourless, white to colourless crystalline solid. The compound is non-polar and therefore has a low solubility in water and an affinity for organic substances. It is also thermally sensitive at temperatures over 50 °C and decomposes at 130 °C (NRAAVC 2000; WHO 2004).
Technical grade chlorpyrifos has a minimum purity of 940 to 990 g/kg. It is a white to light yellowish brown crystalline solid with a mild mercaptan odour. Commercial formulations of chlorpyrifos are generally produced as a concentrated emulsion, liquid, wettable powder, dust, solid bait or granules (NRAAVC 2000).
Chlorpyrifos has been widely used in the Australian agricultural industry since the mid-1960s as it is reportedly less harmful to beneficial insects and is a useful tool in insecticide resistance management programs (NRAAVC 2000). It is used to control insects in soil and on crop foliage including fruit (pome, stone and citrus fruit, strawberries, figs, pineapples, kiwifruit and bananas), nuts, vines, vegetables (potatoes, asparagus), grains (rice, cereals, maize, sorghum), cotton, mushrooms, sugar cane, turf and ornamental plants (NRAAVC 2000). In industrial/commercial and domestic buildings chlorpyrifos is used to control termites, cockroaches, spiders, ants, mosquitoes and fleas and is generally sprayed in the sub-floor region during construction or applied around the building. It is also registered for use in dog and cat flea collars, sprays and shampoos. While the number of products containing chlorpyrifos changes on a yearly basis[1], in 2000 there were 164 products registered in Australia that contained chlorpyrifos (NRAAVC 2000).
In contrast to Australia, the US banned all domestic use of chlorpyrifos in 2001.
Chlorpyrifos is persistent in the environment with a half-life in soil reported to range from 3356 days for soil-incorporated applications (Tomlin 2003) to 462 days in Australian soil under conditions similar to the application of products on soil for termite control (Baskaran et al. 1999).
No previous HIL is available for chlorpyrifos (NEPC 1999). It is noted, however that review of chlorpyrifos by Taylor & Di Marco (2003) derived a health-based soil investigation level (residential) of 80 mg/kg on the basis of a threshold toxicity reference value of 0.003 mg/kg/day (noted to be derived from US EPA), 100% oral bioavailability, soil ingestion only, and an assumption that exposures from soil contribute (by default) 20% of the reference value.
Insufficient data is available to adequately define the bioavailability of chlorpyrifos, hence a default approach of assuming 100% oral bioavailability has been adopted in the derivation of an HIL. It is noted that a site-specific assessment of bioavailability can be undertaken where required.
Limited data is available on dermal absorption of chlorpyrifos. Review by APVMA (2009) identified that in acute animal studies, dermal absorption has been shown to be low. In human volunteers, dermal absorption was estimated to be 1.35% of the applied dose (NRAAVC 2000). Dermal absorption of chlorpyrifos in soil (not in solution) is expected to be lower. The assessment of occupational exposures by NRAAVC (2000), as confirmed by APVMA (2009), has adopted a dermal absorption value of 3%. This has been adopted in the derivation of HILs.
The inhalation exposure pathway is expected to be of significance during and immediately after the application of products containing the product. In these cases chlorpyrifos may be present in the vapour phase as well as sorbed to particulates (ATSDR 1997). An Australian study by Beard et al. (1995) demonstrated that airborne exposures to pesticides in the community can be substantial and are largely related to residential use of pesticides rather than agricultural applications. These issues should be considered on a site-by-site basis.
For the assessment of chlorpyrifos as a soil contaminant (no product application considered), the compound is not considered sufficiently volatile to be of significance and inhalation exposures associated with particulates outdoors and indoors are expected to be of less significance than ingestion of soil. While likely to be negligible, potential inhalation exposures associated with dust have been considered in the HIL derived.
Information relating to the potential for plant uptake of chlorpyrifos is mixed. ATSDR (1997) notes that some research has shown that only very small levels of chlorpyrifos are taken up by plant roots, translocated, or metabolised by plant tissues. However, other researchers have found that soil-applied doses of chlorpyrifos are transported to foliage. APVMA (2009) notes that absorption and translocation of foliar deposits of chlorpyrifos is very low, with the bulk dissipating through volatilisation. Absorption by roots from the soil is also poor. This is further supported by studies presented by JMPR (1972) that show that the uptake of chlorpyrifos or its degradation products is insignificant through the foliage or roots. Only through the use of specialised techniques has plant uptake of chlorpyrifos been significant.
Chlorpyrifos has the potential to strongly adsorb to soil and sediments (based on log Koc of 3.73 from ATSDR (1997)) and has low water solubility. Hence the potential for chlorpyrifos to be present in soil solution, and subsequent uptake by plants, is considered to be low.
On the basis of the available information, plant uptake into edible fruit and vegetable crops is considered low and has not been considered in the derivation of soil HILs.
Background intakes were evaluated in more detail by Taylor & Di Marco (2003), where data (from Australia where relevant) for food, water and air were considered. Background intakes were estimated to range from 0.81 µg/kg/day for adults and infants to 1 µg/kg/day for toddlers. Dietary intakes of 0.63 µg/kg/day for toddlers (based on older surveys) were higher than currently reported. Current data on intakes from food and air (most significant pathways considered) include:
Other sources of exposure may be associated with house dust, though as there is limited data available to quantify exposures related to the presence of chlorpyrifos in house dust, it has not been included in this evaluation. It is noted that the derivation of the soil HIL considers ingestion of both soil and dust.
Consideration of intakes derived from food and air suggests background intakes may be approximately 1.6 µg/kg/day, which comprise approximately 50% of the recommended TRV. Review of dietary intakes by APVMA (2009), based on a conservative estimate of chemical residues in food, indicated that intakes may comprise up to 55% of the TRV, similar to the estimate presented on the basis of the above.
As chlorpyrifos remains in use in Australia it is reasonable, based on the above, to consider background intakes to be more than negligible. Based on the estimates, intakes derived from dietary and atmospheric sources have been estimated to be approximately 1.6 µg/kg/day (50% of the TRV) and have been considered in the derivation of soil HILs.
The International Agency for Research on Cancer (IARC) has not classified chlorpyrifos as to carcinogenicity and US EPA has classified it as Group D—not classified for carcinogenicity
Limited data is available on the carcinogenicity of chlorpyrifos. However, chlorpyrifos has not been identified as carcinogenic in long-term animal studies, and was not genotoxic in a wide range of assays (NRAAVC 2000; APVMA 2009). On this basis, the assessment of exposures to chlorpyrifos on the basis of a threshold approach is appropriate.
The following are available from Level 1 Australian and International sources:
Source | Value | Basis/Comments | ||
Australian | ||||
ADWG (NHMRC 2011) | ADI = 0.003 mg/kg/day | Current ADWG (NHMRC 2011, established in 1998) of 0.01 mg/L based on a NOEL of 0.03 mg/kg/day for plasma cholinesterase inhibition from a 28-day volunteer study in humans, and an uncertainty factor of 10. | ||
OCS (2012) | ADI = 0.003 mg/kg/day | The ADI of 0.003 mg/kg/day (set in December 1998) is based on the same approach as noted in the ADWG above. | ||
NRAAVC (2000) and APVMA (2009) | ADI = 0.003 mg/kg/day | The APVMA (2009) review provided an updated toxicology assessment for chlorpyrifos. The review considered the range of threshold values derived by different countries with respect to the selection of relevant end points and other factors (including sensitive sub-populations such as children). The review did not identify any new studies that would result in changes to the toxicological end points selected for either public or occupational health assessments. The end points used in the NRA (2000) review were considered to be valid. No toxicological effects were observed at doses lower than those that resulted in inhibition of plasma cholinesterase activity in a human volunteer study. On the basis of this effect in humans at a dose of 0.1 mg/kg/day, with no effects seen at 0.03 mg/kg/day, the ADI at 0.003 mg/kg/day was established, with a 10-fold safety factor used to account for inter-individual variability. | ||
International | ||||
WHO (2011) and JMPR (1983, 2000) | ADI = 0.01 mg/kg/day | ADI adopted in derivation of the current WHO DWG and JMPR (1983, 2000) is based on a NOAEL of 0.1 mg/kg/day based on effects of chlorpyrifos on brain acetylcholinesterase activity in animal studies, and erythrocyte acetylcholinesterase inhibition in human subjects, and an uncertainty factor of 10. Review of this data by APVMA (2009) noted that both of these measures of toxicity are less sensitive than the inhibition of plasma cholinesterase activity, and hence the JMPR ADI is higher (i.e. less conservative) than that set by the OCS. | ||
ATSDR (1997) | Oral MRL = 0.001 mg/kg/day | Chronic oral MRL based on a NOAEL for acetylcholinesterase inhibition in rats exposed to 0.1 mg/kg/day of chlorpyrifos in feed for 2 years, and an uncertainty factor of 100. | ||
US EPA (IRIS 2012) | Not available
| The previous evaluation (oral RfD of 0.003 mg/kg/day) was withdrawn by the US EPA in 2011. No new evaluation is available. | ||
The ADI of 0.003 mg/kg/day identified and considered current in the most recent review by APVMA (2009) and NRA (2000) is consistent with that considered in the derivation of the ADWG (NHMRC 2011) and listed in the ADI List (OCS 2012). The value is considered relevant for the derivation of a soil HIL in Australia.
No dermal or inhalation-specific studies or data are available. For the presence of chlorpyrifos in soil, it is considered appropriate to consider use of the available ADI for all pathways of exposures.
On the basis of the discussion above, the following toxicity reference values (TRVs) have been adopted for chlorpyrifos in the derivation of HILs:

On the basis of the above, the following HILs have been derived for chlorpyrifos (refer to Appendix B for equations used to calculate the HILs and Appendix C for calculations):
HIL Scenario | HIL (mg/kg) | Percentage Contribution from Exposure Pathways | |||
Ingestion of Soil/Dust | Ingestion of Home-grown Produce | Dermal Absorption of Soil/Dust | Inhalation (dust) | ||
Residential A | 160 | 72 | -- | 28 | <1 |
Residential B | 340 | 38 | -- | 62 | <1 |
Recreational C | 250 | 55 | -- | 45 | <1 |
Commercial D | 2000 | 31 | -- | 69 | <1 |
-- Pathway not included in derivation of HIL
APVMA 2009, Chlorpyrifos, Preliminary Review Findings Report on Additional Residues Data, a reconsideration of the active constituent approvals of chlorpyrifos, the registration of products containing chlorpyrifos and their associated labels, APVMA, August 2009.
ATSDR 1997, Toxicological profile for Chlorpyrifos, Agency for Toxic Substances and Disease Registry (ATSDR), September 1997.
Baskaran, S, Kookana, RS, & Naidu, R 1999, ‘Degradation of bifenthrin, chlorpyrifos and imidacloprid in soil and bedding materials at termiticidal application rates’, Pesticide Sciences, vol. 55, pp. 12221228.
Beard, J, Westley-Wise, V, & Sullivan, G 1995, ‘Exposure to pesticides in ambient air’, Australian Journal of Public Health, vol.19, pp. 357362.
EA 2001, Air toxics and indoor air quality in Australia, State of knowledge report, Environment Australia, 2001, available from http://www.environment.gov.au/atmosphere/airquality/publications/sok/chapter7.html.
FSANZ 2011, The 23rd Australian Total Diet Study, Food Standards Australia and New Zealand.
JMPR 1972, Chlorpyrifos, WHO Pesticides Residues Series 2, available from http://www.inchem.org/documents/jmpr/jmpmono/v072pr10.htm.
JMPR 1983, FAO/WHO (1983), Pesticide residues in food — 1982 evaluations, World Health Organization, Joint FAO/WHO Meeting on Pesticide Residues, (FAO Plant Production and Protection Paper 49), Geneva.
JMPR 2000, FAO/WHO (2000), Pesticide residues in food — 1999 evaluations, Part II — Toxicological, World Health Organization, Joint FAO/WHO Meeting on Pesticide Residues, (WHO/PCS/00.4), Geneva.
NEPC 1999, Schedule B (7a), Guideline on Health-Based Investigation Levels, National Environment Protection (Assessment of Site Contamination) Measure, National Environment Protection Council, Australia.
NHMRC 2011, National water quality management strategy, Australian drinking water guidelines, National Health and Medical Research Council, Australia.
NRAAVC 2000, The NRA Review of Chlorpyrifos, vol. 1, NRA Review Series 00.5, Canberra, Australia.
OCS 2012, ADI List, Acceptable Daily Intakes for Agricultural and Veterinary Chemicals, current to 31 March 2012, Australian Government, Department of Health and Ageing, Office of Chemical Safety (OCS), available from: http://www.health.gov.au/internet/main/publishing.nsf/content/E8F4D2F95D616584CA2573D700770C2A/$File/ADI-apr12.pdf.
Tomlin, CDS (ed) 2003, The e-Pesticide Manual: a world compendium Chlorpyrifos, 13th edn, PC CD-ROM, Version 3.0, 200304, British Crop Protection Council, Surrey, UK.
US EPA (IRIS 2012), data and information available from the Integrated Risk Information System, an online database, available from http://www.epa.gov/iris/.
Taylor, S & Di Marco, P 2003, ‘Health-Based Investigation Level of Chlorpyrifos’, presented in proceedings of the Fifth National Workshop on the Assessment of Site Contamination, 2003.
WHO 2004, Chlorpyrifos in Drinking Water, Background Document for Development of WHO Drinking Water Quality, World Health Organization, Geneva.
WHO 2011, Guidelines for drinking-water quality, 4th edn, World Health Organization, Geneva, available from http://www.who.int/water_sanitation_health/dwq/chemicals/en/index.html.
Several comprehensive reviews of bifenthrin in the environment and its toxicity to humans are available and should be consulted for more detailed information not presented in this summary (ATSDR 2003; US EPA 1999; Fecko 1999; Taylor & Di Marco 2003). The following provides a summary of the key aspects of bifenthrin that are relevant to the derivation of a soil HIL.
Bifenthrin is the common name for the compound (2-methyl-1, 1-biphenyl-3-y1)-methyl-3-(2-chloro-3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylate. It is referred to as a ‘third generation’ synthetic pyrethroid insecticide and is known to be more stable and persistent in the environment and have a greater insecticidal activity than previously synthesized pyrethroid compounds (Taylor & Di Marco 2003). Pure bifenthrin is a crystalline or waxy solid which is off-white to pale tan in colour.
Bifenthrin is used in the agricultural industry to control insects in a number of crops and to protect stored grains. It is also used in domestic and commercial settings as a barrier to repel or kill insects such as termites (Taylor & Di Marco 2003).
No previous HIL is available for bifenthrin (NEPC 1999). It is noted, however, that review of bifenthrin by Taylor & Di Marco (2003) derived a soil investigation level (residential) of 300 mg/kg on the basis of a threshold toxicity reference value of 0.01 mg/kg/day (noted to be derived from the Therapeutic Goods Administration), 100% oral bioavailability, soil ingestion only, and an assumption that exposures from soil contribute (by default) 20% of the reference value.
Insufficient data is available to adequately define the bioavailability of bifenthrin, hence a default approach of assuming 100% oral bioavailability has been adopted in the derivation of an HIL. It is noted that a site-specific assessment of bioavailability can be undertaken where required.
Insufficient data is available on the dermal absorption of bifenthrin from soil. Hence the default value of 0.1 (10%) suggested by US EPA (1995) for pesticides has been adopted in the derivation of HILs.
It is noted that review by ATSDR (2003) considered the limited human and animal data associated with dermal application of pyrethroids. Dermal absorption values in the range of 0.5% to 1.8% were identified. Hence the adoption of 10% is considered conservative.
Bifenthrin is not considered sufficiently volatile to be of significance and inhalation exposures associated with particulates outdoors and indoors are expected to be of less significance than ingestion of soil. While likely to be negligible, potential inhalation exposures associated with dust have been considered in the HIL derived.
Limited information is available on the potential for plant uptake of bifenthrin. ATSDR (2003) notes that in soils, pyrethrins adsorb strongly and do not leach appreciably into groundwater. These compounds are not considerably taken up by the roots of vascular plants; however, they are deposited upon the leafy region of vegetation following spraying.
Where the application of the product is not of concern, there is limited potential for bifenthrin to be present in soil solution, and available for plant uptake, due to its strong adsorption to soil and its limited solubility.
On this basis, the potential for plant uptake into home-grown fruit and vegetable crops is not considered to be significant and has not been considered in the derivation of a soil HIL.
Background intakes were evaluated by Taylor & Di Marco (2003). No Australian data was identified and intakes from water, food, air, consumer products and soil were assumed to comprise 20% of the adopted ADI, resulting in background intakes from sources other than soil as 80%.
Synthetic pyrethroid pesticides were included in The 23rd Australian Total Diet Survey (FSANZ 2011). Intake associated with the detected residues of bifenthrin for children aged 25 years was 0.072 µg/kg/day, and for children aged 612 years was 0.085 µg/kg/day, similar to the intake estimated for adults.
Limited other data is available in Australia, where a study on bifenthrin in air within a home after termite treatment did not detect bifenthrin concentrations (Richards 2003). Pyrethrins and pyrethroids are used in both indoor and outdoor settings to control insects; therefore, these compounds are frequently detected in the air of homes and buildings after their use. Data from the USA (ATSDR 2003) reported concentrations of pyrethrins in the order of 0.10.3 µg/m3 sometime after application (up to 84 days after application). Intakes by toddlers associated with these concentrations are in the range of 0.06–0.2 µg/kg/day, significantly higher than estimated from dietary intakes. It is noted that if these insecticide sprays are regularly used, indoor air concentrations may be higher.
On the basis of the above, intakes associated with bifenthrin (assuming it comprises 100% of the pyrethrins reported in indoor air in the US) may comprise up to 0.28 µg/kg/day for toddlers, approximately 3% of the recommended oral TRV. For the purpose of establishing an HIL, intakes from other sources has been taken to be 10% of the adopted TRV.
The International Agency for Research on Cancer (IARC) and US EPA have not classified bifenthrin as to carcinogenicity. It is noted that the Joint Meeting on Pesticide Residues (JMPR 1993) has reviewed bifenthrin, which was evaluated as unlikely to pose a carcinogenic hazard to humans.
A summary of health effects and information is presented by Taylor & Di Marco (2003). Limited data is available for the assessment of carcinogenicity, though the available data suggests that bifenthrin was not likely to pose a carcinogenic risk to humans.
On the basis of the available information it is considered appropriate that a threshold doseresponse approach be adopted for bifenthrin. The following are available from Level 1 Australian and International sources:
Source | Value | Basis/Comments |
Australian | ||
ADWG | No evaluation available |
|
OCS (2012) | ADI = 0.01 mg/kg/day | The ADI of 0.01 mg/kg/day based on maternal tremors in a developmental rat study. The value was set in 1992. The ADI is also used by FSANZ (2003). |
International | ||
JMPR (1993) | ADI of 00.02 mg/kg/day | ADI established on the basis of a NOAEL of 1.5 mg/kg/day in a 1-year study in dogs, and a 100-fold uncertainty factor. The study was supported by the same NOAEL in the rat teratology study. ADI presented has been rounded by JMPR. |
WHO | No evaluation available |
|
RIVM (2001) | No evaluation available |
|
ATSDR | No evaluation available |
|
US EPA (IRIS 2012) | RfD = 0.015 mg/kg/day
| US EPA has established an oral RfD of 0.015 mg/kg/day based on a NOEL of 1.5 mg/kg/day associated with tremors in a 1-year dog study, and 100-fold uncertainty factor. |
Based on the available data, the current Australian ADI of 0.01 mg/kg/day is considered current and relevant.
No dermal or inhalation-specific studies or data are available. For the presence of bifenthrin in soil (not during use), it is considered appropriate to consider use of the available threshold reference value for all pathways of exposures.
On the basis of the discussion above, the following toxicity reference values (TRVs) have been adopted for bifenthrin in the derivation of HILs:
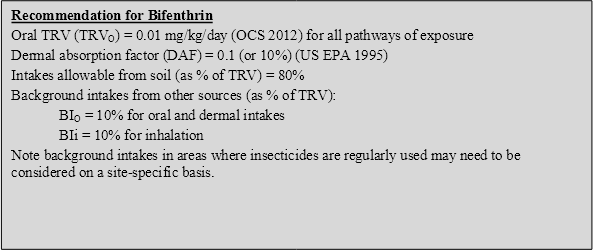
On the basis of the above, the following HILs have been derived for bifenthrin (refer to Appendix B for equations used to calculate the HILs and Appendix C for calculations):
HIL Scenario | HIL (mg/kg) | Percentage Contribution from Exposure Pathways | |||
Ingestion of Soil/Dust | Ingestion of Home-grown Produce | Dermal Absorption of Soil/Dust | Inhalation (dust) | ||
Residential A | 600 | 43 | -- | 57 | <1 |
Residential B | 840 | 16 | -- | 84 | <1 |
Recreational C | 730 | 27 | -- | 73 | <1 |
Commercial D | 4500 | 12 | -- | 88 | <1 |
-- Pathway not included in derivation of HIL
ATSDR 2003, Toxicological Profile for Pyrethrins and Pyrethroids, Agency for Toxic Substances and Disease Registry, September 2003.
Fecko, A 1999, Environmental Fate of Bifenthrin, December 28 1999 last update, (online), available at http://www.pw.ucr.edu/textfiles/bifentn.pdf.
FSANZ 2011, The 23rd Australian Total Diet Study, Food Standards Australia and New Zealand.
JMPR 1993, Pesticide Residues in Food 1992, Toxicological and Environmental Evaluations, Bifenthrin, Report of the joint meeting of the FAO Committee on Pesticides in Agriculture and the WHO Expert Committee on Pesticide Residues, Rome 2130 September 1992, World Health Organization, Geneva.
NEPC 1999, Schedule B (7a), Guideline on Health-Based Investigation Levels, National Environment Protection (Assessment of Site Contamination) Measure, National Environment Protection Council, Australia.
OCS 2012, ADI List, Acceptable Daily Intakes for Agricultural and Veterinary Chemicals, current to 31 March 2012, Australian Government, Department of Health and Ageing, Office of Chemical Safety (OCS), available from: http://www.health.gov.au/internet/main/publishing.nsf/content/E8F4D2F95D616584CA2573D700770C2A/$File/ADI-apr12.pdf
Richards, G 2003, ‘Suspected Pesticide Poisoning: A Back-of-the-envelope Health Risk Assessment’, NSW Public Health Bulletin, vol .14, No 8, pp. 168170.
RIVM 2001, Re-evaluation of human-toxicological Maximum Permissible Risk levels, National Institute of Public Health and the Environment, Bilthoven, Netherlands, available from: http://www.rivm.nl/bibliotheek/rapporten/711701025.html.
Taylor, S & Di Marco, P 2003, ‘Health Based Investigation Level for Bifenthrin in Soil’, proceedings of the fifth National Workshop on the Assessment of Site Contamination, National Environment Protection Council, Environment Protection and Heritage Council, Australia.
US EPA 1995, Technical Guidance Manual, Assessing Dermal Exposure from Soil, US EPA Region 3, December 1995, available from: http://www.epa.gov/reg3hwmd/risk/human/info/solabsg2.htm.
US EPA 1999, Environmental Fate Assessment for the Synthetic Pyrethroids. Environmental Fate Effects Division, United States Environment Protection Authority, www.epa.gov:80/opp00001/SAP/1999/february/pyreth.pdf.
US EPA (IRIS 2012), data and information available from the Integrated Risk Information System, an online database, available from http://www.epa.gov/iris/.
ADI | acceptable daily intake |
ADWG | Australian Drinking Water Guidelines |
AI | adequate intake |
ANZECC | Australia and New Zealand Environment and Conservation Council |
APVMA | Australian Pesticides and Veterinary Medicines Authority |
ATDS | Australian Total Diet Survey |
ATSDR | Agency for Toxic Substances and Disease Registry |
BA | bioavailability |
BI | background intake |
BMD | benchmark dose |
BMDL | Benchmark dose lower confidence limit |
CCME | Canadian Council of Ministers of the Environment |
CICAD | Concise International Chemicals Assessment Document |
CNS | central nervous system |
DAF | dermal absorption factor |
DW | dry weight |
EA | Environment Agency (England and Wales) |
EHC | Environmental Health Criteria |
EPA | Environment Protection Authority |
FSANZ | Food Standards Australia and New Zealand |
GAF | gastrointestinal absorption factor |
HCB | hexachlorobenzene |
HEC | human equivalent concentration |
HED | human equivalent dose |
HIARC | Hazard Identification Assessment Review Committee |
HIL | health investigation level |
HSDB | Hazardous Substances Data Bank |
HSL | health screening level |
IARC | International Agency for Research on Cancer |
IEUBK | Integrated exposure uptake biokinetic model |
IRIS | Integrated Risk Information System |
JECFA | Joint FAO/WHO Expert Committee on Food Additives |
JMPR | WHO/FAO Joint Meeting on Pesticide Residues |
LOAEL | lowest observed adverse effect level |
LOEL | lowest observed effect level |
MF | modifying factor |
MOA | mode (or mechanism) of action |
MRL | maximum residue limit |
MRL | minimal risk level |
NDI | negligible daily intake |
NEPC | National Environment Protection Council |
NEPM | National Environment Protection Measure |
NHMRC | National Health and Medical Research Council |
NOAEL | no observable adverse effect level |
NOEL | no observable effect level |
NSW DECC | New South Wales Department of Environment and Climate Change |
OCS | Office of Chemical Safety |
POP | persistent organic pollutant |
PTDI | provisional tolerable daily intake |
PTMI | provisional tolerable monthly intake |
PTWI | provisional tolerable weekly intake |
RAIS | Risk Assessment Information System |
RDI | recommended daily intake |
REL | reference exposure level |
RfC | reference concentration |
RfD | reference dose |
RME | reasonable maximum exposure |
SF | slope factor |
TC | tolerable concentration |
TD | tumorigenic dose |
TDI | tolerable daily intake |
TRV | toxicity reference value |
UF | uncertainty factor |
UL | upper limit |
UR | unit risk |
US EPA | United States Environmental Protection Agency |
WHO | World Health Organization |
WHO DWG | World Health Organization Drinking Water Guidelines |
[1] Refer to APVMA Public Chemical Registration Information System (PUBCRIS) for current information on products that contain chlorpyrifos (http://services.apvma.gov.au/PubcrisWebClient/welcome.do )