
![]()
![]()



![]()
![]()


 Page
Page
1 Introduction
1.1 Objectives
1.2 Overview of Schedule B4
1.3 Introduction to quantitative health risk assessment in contaminated land decision-making
1.4 Site assessment process and terminology
1.4.1 Health investigation levels
1.4.2 Conceptual site model
1.4.3 The tiered approach
2 The Australian risk assessment framework
2.1 The enHealth framework
2.2 Risk assessment framework for contaminated sites
2.3 Fundamentals of the risk assessment approach
2.3.1 Issues identification
2.3.1.1 Planning and scoping
2.3.1.2 Problem formulation
2.3.2 Exposure pathways
2.3.3 Conceptual site model
2.4 The tiered approach
2.4.1 Fundamentals of the tiered approach
2.4.1.1 Tier 1
2.4.1.2 Tier 2
2.4.1.3 Tier 3
2.4.2 General risk assessment assumptions
2.4.3 Risk assessment endpoints
2.4.4 Deterministic versus probabilistic estimates
3 Data collection and data evaluation
3.1 Data collection
3.2 Source variables
3.2.1 Organic speciation
3.2.2 Metals speciation
3.2.3 Background concentrations
3.2.4 Vapour and particulate (dust) sampling
3.3 Exposure pathway variables
3.3.1 Organic carbon content
3.3.2 Other key pathway parameters
3.4 Data evaluation
3.4.1 Data quality assessment and data quality objectives
3.4.1.1 Analytical methods
3.4.1.2 Data quality objectives
3.4.1.3 Limits of detection
3.4.1.4 Density and distribution of samples
3.4.2 Three dimensional source definition
3.4.3 Refining the exposure pathways
3.4.4 Tier 1 screening
4 Exposure assessment
4.1 Introduction
4.2 Exposure settings
4.2.1 Defining model inputs to represent the contaminant source
4.2.2 Exposure pathway input values
4.2.3 Exposed population input values
4.3 Exposure point concentrations
4.4 Exposure point concentrations volatiles
4.4.1 Introduction
4.4.2 Indoor air concentrations:
4.4.3 Outdoor air concentrations
4.4.4 Finite and infinite sources
4.4.5 Biodegradation
4.4.6 Vapours from non-aqueous phase liquids
4.5 Exposure point concentrations particulates
4.6 Exposure point concentrations food consumption
4.6.1 Fruit and vegetable consumption
4.6.2 Poultry, meat and fish consumption
4.7 Estimation of contaminant intake
4.7.1 Introduction
4.7.2 Ingestion intakes
4.7.3 Dermal intakes
4.7.4 Inhalation intakes
4.8 Specific considerations in exposure modelling
4.8.1 Blood lead modelling
4.8.2 Bioavailability and bioaccessibility
4.8.3 The approach for petroleum hydrocarbons
5 Toxicity assessment
5.1 Introduction
5.1.1 Sources of toxicity information
5.1.2 Sources of physical and chemical data
5.2 Hazard identification
5.2.1 Acute effects
5.2.2 Chronic threshold effects
5.2.3 Chronic cancer effects
5.3 Doseresponse assessment
5.3.1 Overview
5.3.2 Threshold toxicity reference values
5.3.3 Cancer toxicity reference values
5.4 Other considerations in toxicity assessment
5.4.1 Absence of information
5.4.2 Early-life susceptibility
5.4.3 Metal speciation
6 Risk Characterisation
6.1 Overview
6.2 General risk characterisation principles
6.3 Risk estimation
6.3.1 Threshold risk estimation
6.3.2 Non-threshold risk estimation
6.4 Risk evaluation
6.4.1 Threshold risk evaluation
6.4.2 Non-threshold risk evaluation acceptable level of cancer risk
6.5 Risk evaluation of mixtures
6.6 Uncertainty and sensitivity analysis
6.6.1 Uncertainty analysis
6.6.2 Sensitivity analysis
7 Risk communication and management
7.1 Risk communication
7.2 Risk management
8 Bibliography
9 Appendix 1: Structure of a risk assessment report
9.1 Introduction
9.2 General
9.3 Key principles
9.3.1 Overview
9.3.2 Issues identification
9.3.3 Data collection and evaluation (development of a conceptual site model)
9.3.4 Exposure assessment
9.3.5 Toxicity assessment
9.3.6 Risk characterisation
9.3.6.1 Overview
9.3.6.2 Uncertainty
9.3.6.3 Sensitivity analysis
10 Glossary
11 Shortened forms
The objectives of this revised Schedule B4 guideline to site-specific health risk assessment methodology of the National Environment Protection (Assessment of Site Contamination) Measure 1999 (the NEPM) are to:
The intended audience for this Schedule includes policy makers, risk practitioners and regulators.
This guidance is not intended to be used to assess the risks from occupational exposure to substances that may occur in an occupational setting or workplace. These risks are dealt with under work health and safety legislation and associated guidelines.
The enHealth Committee (enHealth) of the Australian Health Protection Committee (enHealth 2012a) defines risk assessment as ‘the process of estimating the potential impact of a chemical, physical, microbiological or psychosocial hazard on a specified human population or ecological system under a specific set of conditions and for a certain timeframe’.
Quantitative (health) risk assessment is a step-wise process used to inform and assist the contaminated land decision-making process by modelling the dose or exposure of humans to observed site contamination. It is used to estimate, in a way that is adequately protective of health, the potential for site contamination to have an adverse effect on the health of those potentially exposed to it (referred to as exposed populations). This is achieved by modelling the dose that an individual may receive through incidental exposure to contaminated soil and/or water as a result of everyday activities. This estimated dose can then be compared against doses that are considered to result in no observable adverse impact to health, as published by authoritative bodies and health protection agencies.
The quantitative risk assessment process, in the context of this guidance, is primarily designed to evaluate the long-term or chronic risks to exposed populations from contamination in the environment. Short-term or acute risks can be dealt with using similar means though normally this is not necessary, because contamination that is severe enough to pose acute risks is sufficiently obvious that a complex assessment is not required.
There are two different approaches to risk assessment, which can be referred to as ‘forward’ and ‘backward’ assessments. In a forward assessment, the risks associated with a measured contaminant concentration are estimated by comparing an estimated dose that a receptor (i.e. a person) may receive with a dose considered to result in no significant observable impact to health. It is possible to undertake the modelling in reverse (a backward assessment), starting with the dose considered to result in no significant observable impact to health, and back-calculating ‘tolerable’ contaminant concentrations at the site. These concentrations can then be used to define management requirements or as remediation targets or site-specific clean-up criteria.
The general site assessment process for contaminated land is described in Schedule A of this NEPM. Once the need for an assessment is triggered, a preliminary site investigation should be conducted using guidance outlined in Schedule B2. The objectives of the preliminary investigation are to identify the potential contaminants of concern, the areas of potential contamination and the potentially affected media (i.e. soil, water, sediment and air). The preliminary investigation report should clearly identify any significant data gaps and include an assessment of the accuracy of the information collected.
Where there is potential for soil and/or groundwater contamination to be present, then a detailed site investigation should be conducted. This involves the collection of soil, groundwater and soil vapour samples for field and laboratory analysis, and should be conducted using appropriate guidance such as that outlined in Schedule B2 and Schedule B3.
The sample analysis results are then compared against appropriate guidance values, such as HILs (after appropriate checking of the reliability of the data).
HILs and health screening levels (HSLs) are defined as ‘the concentration of a contaminant above which further appropriate investigation and evaluation will be required’. They are defined for specific land uses and will not necessarily be protective for other land uses. There are a number of assumptions (for example, relating to site geology or the nature of exposure) built into the HILs and HSLs, and careful review should be undertaken to determine whether these assumptions are applicable on a given site. The absence of an HIL for a specific contaminant does not indicate that no potential risk is posed; rather that a site-specific risk assessment may be required.
The HILs are presented in Schedule B7. HSLs have been developed separately by CRC CARE (Friebel & Nadebaum 2011) and may be adopted where applicable (for further information refer to Schedule B1). Levels marginally in excess of the HILs and HSLs (where applicable) do not imply unacceptability or that a health risk is likely to be present. Similarly, levels less than the HILs may not imply acceptability or that a health risk does not exist for a sensitive sub-population (for example, people with pre-existing illness and people with pica (relatively common in some groups with severe or profound intellectual disability)). These issues would need to be addressed in site-specific assessment. Subject to an appropriate investigation and assessment process, a decision not to take further action or to take further action may be justifiable.
HILs and HSLs (where applicable) are not intended to be clean-up levels. The decision on whether clean-up is required and if so, to what extent, should be based on site-specific assessment. Health risk assessment is one aspect of making such a decision though other considerations such as practicality, timescale, effectiveness, cost and durability are also important.
A conceptual site model (CSM) is a representation of site-related information regarding contaminant sources, receptors and the exposure pathways between those sources and receptors. The development of a CSM is an essential part of all site assessments and provides the framework for identifying how the site became contaminated and how potential receptors may be exposed to contamination either in the present or in the future.
In order to commence a risk assessment, it is necessary to develop a preliminary CSM. Factors to be considered include:
Additional information is presented in Schedule B2.
The human health risk assessment process for assessment of site contamination is generally undertaken in stages or ‘tiers’ involving progressively more detailed levels of data collection and analysis. In this guidance, the tiers are referred to as Tier 1, Tier 2 and Tier 3. The approach provides for assessment at a level of complexity that is appropriate for the problem under consideration; the degree of health protection achieved is equal at each tier. As the amount of data and assessment detail increases and the conceptual understanding of site conditions (that is, the CSM) is refined, the level of uncertainty decreases.
A risk assessment progresses from Tier 1 to Tier 2 when uncertainty and risks at Tier 1 may be unacceptable and further assessment is needed. Progression from Tier 2 to Tier 3 is similarly driven. Tier 3 provides more detailed and specific focus on risk-driving factors. It should be noted that the activities within the tiers may vary depending on the scale and complexity of the project.
The Environmental Health Committee (enHealth) provides national leadership on human health issues from environmental hazards, coordinates national policies and programs, and provides a pivotal link between international and environmental health stakeholders in Australia. It is also responsible for the implementation of the National Environmental Health Strategy. Additional information on enHealth, including access to its publications, is available via the Department of Health and Ageing website:
http://www.health.gov.au/internet/main/publishing.nsf/Content/ohp-environ-enhealth-committee.htm.
In 2004, enHealth developed Environmental Health Risk Assessment - Guidelines for assessing human health risks from environmental hazards (enHealth 2004) to provide a national approach to undertaking environmental health risk assessments and updated these guidelines in 2012 (enHealth 2012a). The guidelines present a general environmental health risk assessment methodology that has been adopted nationally to evaluate risks and establish standards for the protection of human health and the environment.
With respect to assessment of risks from contaminated land, the guidelines draw on other documents as follows:
The enHealth guidelines adopt a framework for evaluating risks that was developed by and for environmental health agencies, including the World Health Organization (WHO) and the United States Environmental Protection Agency (US EPA). As discussed in Section 2.2, the framework presented in enHealth forms the basis for the risk assessment framework adopted in this schedule.
The framework comprises the following components:
The risk assessment process for contaminated land is intended to achieve the following objectives:
The assessment framework was originally outlined by NEPC in the Assessment of Site Contamination National Environmental Protection Measure (NEPC 1999). The major difference between the framework originally outlined by NEPC and that of enHealth is the first step in the assessment process, which in enHealth (2012a) is referred to as ‘issues identification’. The term ’issues identification’ is intended to establish the context for the risk assessment by a process of identifying the concerns that need to be addressed, such as ‘what is causing the identified concern?’, and ‘why is the concern an issue?’. Inclusion of the ‘issues identification’ as an explicit need is consistent with US conclusions that increased attention to scoping and planning of risk assessment is necessary (NRC 2008).
This revised schedule has adapted the enHealth framework with additions intended to provide guidance specific to a contaminated land context. Consistent with guidance provided in enHealth (Section 16.1 of enHealth (2012a)), in the assessment of contaminated sites, this Schedule takes precedence over the enHealth framework, and documents referenced therein, where there are contradictions. It is to be noted that the enHealth framework has a wider remit than the assessment of contaminated sites only, and some elements of the guidance are not relevant in a contaminated sites context.
The framework followed in this Schedule is illustrated in Figure 1. Detailed guidance for each stage of the process is provided in the body of this Schedule.
All risk assessments should be fully documented in order to ensure transparency, consistency in decision-making and ease of understanding by interested parties.
This means it should be supported with references to policy, scientific literature and other sources, including expert opinion. Jurisdictions may also require that a risk assessment is subject to review and revision should significant new information (that has the potential to change the objectives or outcome of the risk assessment) become available.
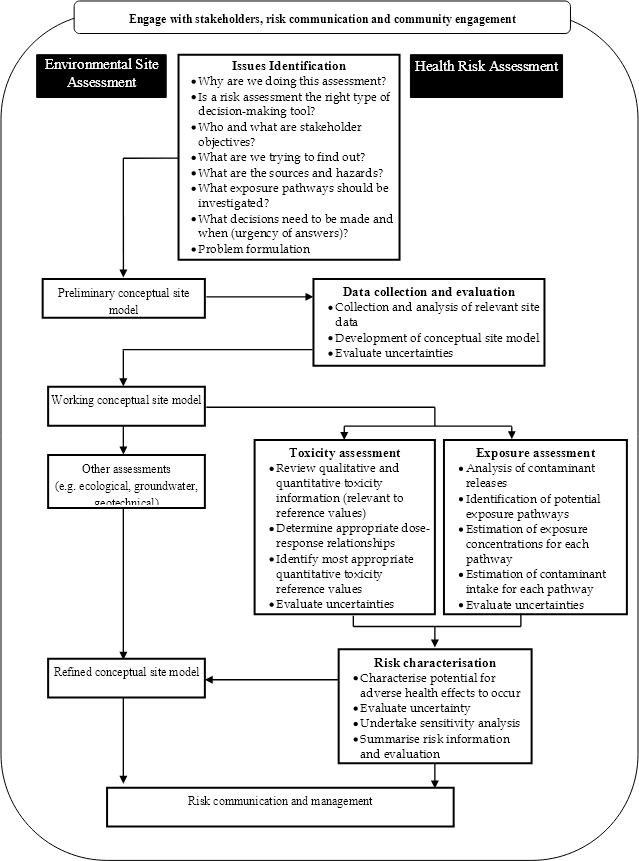
Figure 1. Risk assessment framework for contaminated sites
The issues identification stage of a contaminated land risk assessment is fundamental to the production of a useful output. Issues identification is a process of communication between stakeholders in the project, and its scope and complexity depends upon the scale of the project and the issues being dealt with. Issues identification covers both a planning and scoping phase and a problem formulation stage.
In the planning and scoping phase, a team of decision-makers, stakeholders and risk assessors identifies the issue (or concern, problem or objective) to be assessed and establishes the goals, breadth, depth and focus of the assessment. The primary product of planning and scoping is a statement, with an explanation of why the assessment is being performed and what it will include and exclude, that is, how comprehensive it will be (NRC 2008).
Stakeholders in a contaminated land risk assessment are likely to be a subset of the following groups:
The planning and scoping phase should be undertaken before work begins on the risk assessment. The steps recommended are:
Frame the answers to the following questions and discuss them with the stakeholders:
With the outcome of the consultation known, determine the objectives for the risk assessment. Where not all the stakeholders have been consulted, consideration of their likely objectives should be included.
The problem formulation stage is where discussions are conducted on how to conduct a risk assessment that covers the matters identified in the planning and scoping stage. Two critical products of the problem formulation stage are a conceptual model that explicitly identifies the stressors, sources, receptors, exposure pathways and potential adverse human health effects that the risk assessment will evaluate, and an analysis plan (or work plan) that outlines the analytic and interpretive approaches that will be used in the risk assessment (NRC 2008).
The fundamental concept of risk assessment is that there should be an exposure pathway linking the source of contamination and the exposed population. Where this linkage exists, an assessment of the nature and significance of the exposure pathway is required to determine the level of risk.
A key concept behind all risk assessments is the definition of a suitable CSM specific to each site. The CSM describes the source(s) of contamination, the pathway(s) by which contaminants may migrate through the various environmental media, and the populations (human or ecological) that may potentially be exposed.
A detailed CSM should include information on the following (on- and off-site as relevant):
Consideration of preferential migration pathways is an essential part of the development of the CSM where volatile and/or dissolved contaminants are present in the subsurface (refer to Schedule B2 and US EPA (2012a) for further information).
A CSM is generally a written description of the site that is accompanied by a schematic, graphical interpretation that depicts what is known or has been inferred about the site. It can also be presented as a flow diagram, as shown by the example in Figure 2. It can be simple or complex, with a more complex example shown in Figure 3.
Primary | Secondary | Transport | Exposure | Exposed Population |
|
| |||
Figure 2. Example Conceptual Site Model flow diagram (modified from ASTM, 1995)
A conceptual site model can inform the development of and be incorporated into the detailed scope for a human health risk assessment such as is shown in Figure 3. This example deals with the scoping of a risk assessment for hazardous air pollutants (HAP) and persistent, bioaccumulative and toxic pollutants (PBT)
The schema identifies the sources, contaminants of concern (stressors), exposure pathways, potential receptors, and adverse human health effects that the risk assessment will address. The pathways presented are for illustrative purposes only and are not relevant to any specific scenario.
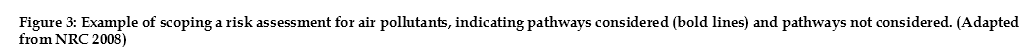
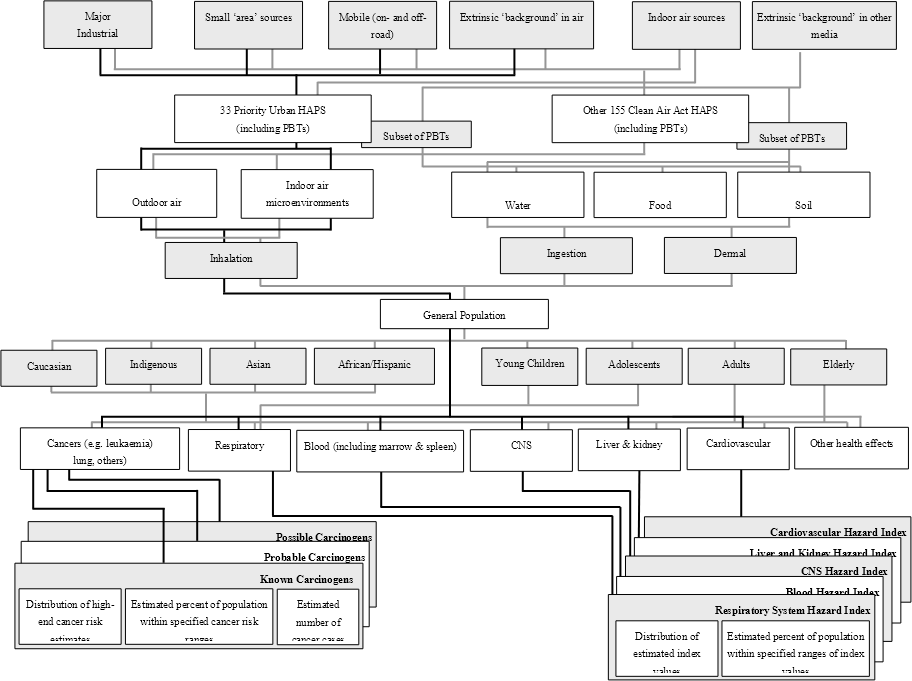
Sources
|
Stressors
|
Pathways/ Media
|
Routes
|
Populations
|
Endpoints (Specific non-cancer target
|
Metrics (HAP-specific and cumulative (e.g. by cancer type, weight of evidence, by target organ-specific hazard index) by State |
The Tier 1 (or screening level) assessment is the first stage of assessment at the site. It includes a comparison of known site data with published risk-based guidance levels, such as the HILs. The assessment provides an initial screening of the data to determine whether further assessment is required. HILs are generic Tier 1 guidance values that are designed to be protective of most exposed populations under a variety of circumstances. The assumptions on which the HILs are based (including site conditions and the exposure scenarios) should be understood in order to determine whether the HILs are applicable for a given site. HSLs are similar to Tier 1 values and can be applied in the same manner provided that the associated guidance on suitability and application of the values is considered.
Exceedence of Tier 1 criteria is generally used to define the contaminants that require more detailed assessment at Tier 2. However, chemicals that act via the same toxicological mechanism should be considered carefully before being excluded from Tier 2 assessment. An assessment of the significance of exceedences may be necessary where they are marginal or present over a limited area. Under some circumstances further assessment of contaminants exceeding Tier 1 criteria may not be conducted (e.g. where the extent of the exceedence and cost of remediation is small and further assessment is not cost-effective). Where no further assessment is proposed, a clear and transparent explanation should be provided.
A Tier 1, screening level, assessment is often bypassed where there are no appropriate risk-based guidance levels (including HILs and HSLs), for example, if:
A Tier 2 assessment is typically required when one or more contaminants are present at the site at levels that exceed Tier 1 guidance criteria, if there are no appropriate Tier 1 criteria, or if there are unresolved and significant uncertainties (limiting the reliability of the assessment conducted) identified in the Tier 1 assessment. Tier 2 assessment includes a site-specific risk assessment and the development of site-specific risk-based criteria for comparison with site data. Site-specific risk-based criteria are derived to be adequately protective of human health, but also to take into account site-specific conditions such as relevant exposure pathway linkages to avoid being unnecessarily conservative. Exceedence of Tier 2 criteria may result in a need for a Tier 3 assessment. As with Tier 1 exceedences, an assessment of the significance of exceedences may be necessary where they are marginal or present over a limited area. If Tier 2 criteria are exceeded, but further assessment (or action) is not proposed, the information and logic used to inform the decision should be documented clearly and transparently.
A Tier 3 assessment may be required where exceedence of Tier 2 site-specific risk-based criteria is judged to represent a potentially unacceptable risk to human health. The Tier 3 assessment typically focuses on the risk-driving contaminants in more detail, although studies aimed at reducing the uncertainties inherent in the modelling of exposure pathways are also common at Tier 3. This level of assessment may include statistical methods and mathematical modelling to assess the significance of the site contamination. The collection of additional data, such as soil vapour sampling, ambient air sampling, analysis of dust, biological monitoring and additional site investigations may be needed to support Tier 3 assessments in order to reduce uncertainties.
The tiered approach should provide a process for addressing site contamination methodically, with the level of complexity and cost proportional to the significance of the risk. Increased levels of site-specific data reduce uncertainties inherent in the assessment. It is important to note that for a given site the level of protection of exposed populations should remain the same regardless of the tier of assessment conducted.
Risk assessment is a tool to help risk managers make decisions about contaminated sites. Risk assessment should incorporate an appropriate level of health protection such that sensitive exposed populations are adequately protected. Because of the many uncertainties inherent in risk assessment, it is desirable that quantitative risk assessment methods should overestimate risk to some extent.
Risk assessors should transparently describe the assumptions and uncertainties involved in a risk assessment.
Risk assessors should select exposure model inputs carefully, and consider the reasonableness of the exposure settings when taken together. Some examples of how assumptions may be applied to a model are:
Models should only be used where they are suitable for the exposure scenario being evaluated, and the limitations of the model are adequately addressed and discussed in the assessment report. An overview of contaminant fate and transport modelling is presented in Schedule B2. Specific expertise and experience are required to carry out this type of modelling because of the highly complex nature of most contaminant fate and transport problems.
Some exposure settings and assumptions (as in Tier 1 assessments) may not be realistic for the site under consideration as they are based on generic assumptions and parameters that are not going to be realistic for all sites. A Tier 2 assessment may be used to derive more site-specific values by amendment of the assumptions to reflect actual site conditions.
Where available, data on biodegradation of contaminants and bioavailability of chemicals should be considered (by an appropriately qualified toxicologist), and exposure factors (and assumptions) should reflect the scenarios under consideration. It is not necessary to assume that the HIL assumptions detailed in Schedule B7 prevail under all circumstances; both sites and the exposed populations may be very different from the HIL scenarios and this can and should be accounted for in a Tier 2 or Tier 3 assessment.
There are two different approaches to risk assessment which can be referred to as ‘forward’ and ‘backward’ assessments.
In a forward assessment, site data is assessed to estimate whether the observed contaminant concentrations potentially pose a health risk to exposed populations. Risks are expressed as hazard indices or as increased lifetime cancer risks and these risk estimates are used to support a decision regarding the acceptability of the risk.
In the backward assessment, the starting point of the assessment is the level of risk or exposure that is deemed to be acceptable for the site. The endpoint is an HIL or site-specific risk-based level, which may be used for further assessment or to provide a basis for clean-up.
A deterministic approach means that variables input to an exposure model are expressed as single values or point estimates, which are considered by the assessor to represent the best estimate of the value of the variable. The advantage of this approach is that it is simple and easily understood. Its potential disadvantage is that selection of many point estimates at the upper end of their likely ranges leads to compounding of the uncertainty. Sensitivity and uncertainty analysis are used to overcome this disadvantage and provide increased understanding and clarity on which values are risk-driving; this is itself a useful part of the risk assessment.
Probabilistic techniques can be used to overcome the potential for compounding conservatism and may provide increased understanding of the uncertainty inherent in the assessment results. Monte Carlo analysis and other probabilistic statistical techniques rely on the use of probability distribution functions instead of point estimates to represent the values of variables. A probabilistic exposure model is run over several thousand iterations, and values for each parameter are selected randomly from each distribution at each iteration. The output is also expressed as a probability distribution function.
The advantage of probabilistic methods is that it becomes possible to express the range of potential exposure and risk outcomes. This can aid decision-making by increasing the level of understanding around how likely different risk outcomes may be. The disadvantage is that in order to generate meaningful output, a high level of data and information on the shape of the probability distribution inputs is required. Often this information is not available, and in this case incorrect selection of a probability distribution can introduce error. Some variables are linked (for example, body weight and skin surface area) and if not carefully constructed, probabilistic models may be capable of generating some results that are physically impossible. Another disadvantage of probabilistic risk assessments is the difficulty in explaining this complex approach to the stakeholders who are involved in a site.
In summary, probabilistic techniques are generally not practicable for the majority of assessments at Tier 2, on the basis that there is often insufficient data to support the method. When probabilistic techniques are used, the justification for the probability distribution functions selected as inputs should be clearly given, and dependent variables should be identified and linked.
Data collection entails the acquisition and analysis of information about contaminants at a site that may affect human health and which will be the focus of the risk assessment. The purpose of data collection is to gather data that will improve the CSM and hence enable a more site-specific assessment of risk to be made. Data will relate to not only contaminants (that is, the source) but the physical environment in which they are present (that is, the pathways of exposure). In some instances, it may also be appropriate to gather additional information about the potential exposed populations.
It is recommended that a systematic planning process is used for defining the objectives of a site risk assessment and to develop a sampling plan for the collection and evaluation of representative data to achieve those objectives. The elements of the data collection stage are described in more detail in Schedule B2, including the data quality objective process and the preparation of a sampling and analysis quality plan. The general requirements for data collection in site investigation apply equally to data collected for the purpose of risk assessment. There are a number of issues in data collection that are specific to health risk assessment, which are discussed below.
There are a number of variables commonly required for health risk modelling, which can be measured on a site-specific basis. Site-specific data is preferable to the use of literature or model default values, because environmental variables may have large ranges. Choosing a worst-case value to account for uncertainty adds conservatism to the risk assessment. Consistent selection of worst-case values results in compounding of the conservatism and can result in unrealistic modelled outcomes. Getting site-specific data permits use of realistic values and provides more confidence in the risk assessment outcome.
Several organic compounds are commonly analysed as groups of substances, for example, petroleum hydrocarbons, polycyclic aromatic hydrocarbons (PAHs) and phenols. Such tests may be useful for providing cost-effective estimates of the quantity of contaminant present, but are problematic in risk assessment because toxicity data is generally only available for specific substances. For certain complex mixtures commonly found on contaminated sites (e.g. petroleum hydrocarbons (as discussed in Section 4.8.3), PAHs and PCBs as discussed in Schedule B7, Appendix A2) there are established ways of mitigating this problem, which provide the preferred way of assessing these mixtures. On sites where the contamination can be fully defined by reference to individual specific substances (for which toxicity data is available), it is preferable to assess these specific substances.
Chemical analyses providing detailed breakdowns for groups of organic compounds into individual substances (e.g. PAH), or a limited range of individual indicator compounds plus fractions containing groups of compounds with similar physicochemical properties (e.g. petroleum hydrocarbon fractions) are commercially available. It is commonly necessary to undertake such analyses of mixtures in order to understand the mixture sufficiently to assess risk, because individual compounds within these groups have very different physicochemical and toxicological properties.
The toxicity of metals and metalloids can be highly dependent on the form in which they occur in the environment. A common example is chromium (Cr), where the environmentally stable Cr (III) oxidation state is relatively harmless to humans, but the more oxidised Cr (VI) state is more hazardous to humans. For most metals and metalloids, the HIL assumes that the most toxic form is 100% of the contaminant present in soil. This is very unlikely to be true in most cases, and knowledge of the actual or probable form can be very useful at Tier 2.
Routine metal speciation analysis is commercially available in Australia for species and compounds of arsenic (As), selenium (Se), mercury (Hg), tin (Sn) and lead (Pb), while metal speciation of some other elements is routinely conducted internationally. At sites where potentially toxic metals are present, consideration should be given to whether speciated metal analysis would be relevant to the assessment. Speciated metals data can be useful in refining the risk assessment process. Further discussion on the toxicity of metal species is provided in this Schedule in Section 5.4.3.
It is helpful to understand the prevailing background concentrations of chemical substances, since in some circumstances background concentrations may already exceed screening criteria.
For metal contaminants, if reliable ambient background concentrations cannot be measured, then either the estimation method of Hamon et al. (2004) or collations of ambient background data such as Olszowy et al. (1995) for four Australian cities could be used. To use the Hamon et al. method, it is necessary to establish whether the iron and manganese concentrations of the soil at the site in question are elevated by co-contamination, as the Hamon et al. relationships are based on soils from sites with no known history of contamination other than farming. Further information on these two methods can be found in Section 2.4.9 of Schedule B5b.
Most organic contaminants of interest at contaminated sites have no natural background concentration, though there are notable exceptions, including polycyclic aromatic hydrocarbons. Therefore, ambient background concentrations should either be measured at a suitable reference site, or a default assumption of zero be made for organic contaminants. There are no equivalent models to that of Hamon et al. (2004) available for organic compounds.
Further information on the establishment of background concentrations in soil and groundwater may be found in SA EPA (2008), ANZECC & ARMCANZ (2000) and US EPA (2002b, 2002c).
Direct sampling of ambient air, soil vapour and/or dust can be useful in the risk assessment process, as it provides actual data for inhalation exposure points as opposed to results obtained from fate and transport modelling applied to soil or groundwater data.
Air sampling may be useful in scenarios where human populations are potentially exposed to airborne contaminants over prolonged periods (for example, occupational or residential scenarios).
Australian standards for ambient air quality are provided by the NEPC (2003). Standard methods for sampling and analysis of ambient air and dust are available in various Australian standards (AS 3580 Series; AS 2800-1985; AS 2985-2004; AS 2986-1987; and AS 3640-2009) and US EPA TO Methods (including TO-15 and TO-17, US EPA 1999). Australian guidance on air sampling, analysis, assessment and modelling is available from New South Wales Department of Environment, Climate Change &Water (NSW DECCW, 2010).
Soil vapour sampling guidance is included in Schedule B2 and references therein. Specific guidance on dust sampling for lead is available from US EPA (1995a, 1995b) and ASTM (E1792-03 Standard specification for wipe sampling materials for lead in surface dust, 2011).
The potential for a contaminant to volatilise from soil is strongly influenced by the compound’s soil partition coefficient (Kd), which is a parameter that estimates the potential for the contaminant to adsorb to soil particles. For organic compounds, which sorb most readily to organic carbon within soil, the partition coefficient is a function of the compound’s organic carbon partition coefficient (Koc) and the fraction of organic carbon (Foc) in the soil. The organic carbon partition coefficient represents the chemical partitioning between organic carbon and water in soil. For organic compounds, the relationship between Kd, Koc and Foc is expressed by the following equation:
Kd = Koc x Foc
Fraction of organic carbon is a soil property that can be measured, and which often has a significant effect on the risk assessment outcome for volatile and persistent contaminants. Samples of uncontaminated soil should be tested for Foc such that the concentration of carbon measured is not influenced by the potential presence of organic contaminants. The number of samples required depends upon the inherent variability of the soil being characterised. Sufficient (based on site-specific factors) samples should be taken to represent each soil type being characterised.
Other key physical and chemical parameters that may be required for the risk assessment process include the following:
Data collection for the establishment of these variables is part of the site investigation process. Guidance on site investigation is provided in Schedule B2.
The data evaluation stage is the first stage of the development of the CSM. The level of effort expended and detail of reporting necessary should be proportionate to the amount of data available.
The data evaluation steps include:
Guidance on data quality assessment is presented in Schedule B2 and enHealth guidance (enHealth 2012a) and should be followed when collecting data for risk assessment. Data quality and precision should be such that uncertainty in the risk assessment can be determined and minimised; data quality uncertainties should be explicitly discussed in the uncertainty analysis. Where a number of studies are being combined to provide definition of the source term, particular attention should be paid to the following:
Use of different analytical and sample preparation methods can cause significant differences in results. For example, use of a ‘clean-up’ step (silica gel) in petroleum hydrocarbon analysis removes polar hydrocarbons such as soil humic acids, resulting in a much lower result in many cases. Further information is provided in Section 2.4.4 of Schedule B1. Guidance on analytical methods and their selection is given in Schedule B3.
Data collection is a vital and integral part of the risk assessment process. All data should be collected to meet pre-determined data quality objectives. In many instances, data from a preliminary or detailed site investigation may be available prior to commencing the risk assessment. In such cases, the assessor should determine whether the data quality objectives of any previous investigations are compatible with the objectives of the risk assessment and whether the original data quality objectives have been satisfactorily met. Guidance on the development of data quality objectives is given in Schedule B2.
The detection limit of the analytical method used must be lower than the level at which the contaminant might become a concern (that is, lower than the HIL or Tier 1 screening level).
Under most circumstances, the data should adequately characterise the contamination at the location where the population is likely to be exposed to it. Where sampling density guidelines cannot be followed, the effects of the lack of data on the risk assessment should be considered. Guidance on the development of sampling programs is given in Schedule B2.
Understanding the source is a critical part of risk assessment. There are many factors that control the risk to health—the contaminant concentration is only one of them. The CSM should include a detailed description of the source, bringing together information from the site history, soil and geological information, the depth and extent of the source, and the chemical data. Guidance on developing CSMs can be found in Schedule B2.
Site history should provide information on how the contaminants were released to the soil, and the form in which they were likely to have been released. It may also provide information on the length of time the contaminants have been on the site. This information permits judgements to be made on the likely form and mobility of the contaminants and, together with geological and hydrogeological information, on whether groundwater may be impacted. Site history should also allow judgements to be made on where the contamination is likely to be located.
Contaminants may not be uniformly distributed through the soil profile; they may be associated with a particular soil stratum, such as a layer of imported fill material, or a layer of clay that preferentially adsorbs a contaminant. If the distribution or depth of the contamination is not characterised correctly, the risk can be overestimated or underestimated. The risk of exposure to contaminant vapours derived from soil or groundwater contamination is often driven by the soil type and porosity, the depth of the contamination and the presence of organic carbon in the soil profile. Therefore, by understanding which soil stratum is impacted, key parameters such as soil type, soil depth and Foc can be obtained from the appropriate zone, and a more accurate assessment of the risk can be made.
Site investigation data may either introduce or rule out exposure pathways in the CSM. For example, establishing the groundwater flow direction with improved certainty might show that a pathway to a potentially exposed population does not exist because that exposed population proves to be located upgradient of the source. Detailed guidance is available in Schedule B2.
Guidance on physical hazards such as inhalation of asbestos fibres, risk of fire or explosion from flammable gases and risk of exposure to asphyxiating atmospheres (for example, methane, carbon dioxide, carbon monoxide) is beyond the scope of this Schedule. Guidance on the assessment of asbestos is provided in Schedules B1 and B2. Appropriate guidance should be followed for the assessment of physical hazards.
Exposure pathway assessment should lead to a clear conclusion on which pathways are considered viable and which are not, with reasoning and appropriate evidence. Where viable pathways cannot be assessed in the risk assessment process, appropriate controls for mitigating the risk should be provided and documented.
Tier 1 screening involves comparison of site analytical results with appropriate screening criteria. In Australia, appropriate HILs (including interim HILs for vapour and, where applicable, HSLs for petroleum hydrocarbons and assessment criteria for asbestos) and GILs are used for Tier 1 screening to provide a rapid assessment of whether the site contamination may be of concern with respect to human health. Should contaminant concentrations at a site occur at levels that are below the Tier 1 levels, this implies that for the majority of the people in the population there is no significant health risk from contamination and that remedial action may not be required to protect human health.
For contaminants where HILs are not available, the methodology set out in Schedule B7 could be adopted to derive HIL-equivalent screening criteria. Sources of toxicity reference values should be considered in the same way as has been done for those chemicals with HILs (same hierarchy and other considerations). Exposure scenarios should be used as laid out in Schedule B7. This should only be undertaken by suitably qualified professionals. The resulting values may be used in the same way as HILs. All assumptions and calculations should be transparent, clearly referenced and justified.
Tier 1 values may be adopted from external peer reviewed sources only if the assumptions used to generate the values are equivalent or more conservative than those used to develop the HILs and are suitable for use. In these cases, the relevance of the values and assumptions included in the development of the proposed Tier 1 values should be clearly justified and referenced.
Exceedences of the HILs should be identified and considered. HIL exceedences do not imply that a risk is necessarily present but that further assessment may be justified. HILs are not intended to indicate a clear demarcation between acceptable and unacceptable. Marginal exceedences may not require quantitative Tier 2 risk assessment to conclude that further assessment is not necessary. The magnitude of the exceedence should be considered in the context of the CSM (that is, whether the exposure pathways are plausible and whether exposure will result in harm).
Background concentrations may also be an important consideration at the Tier 1 screening stage. If it can be clearly demonstrated that site concentrations are consistent with natural regional background levels (natural or anthropogenic), this can provide evidence that site contamination has not significantly increased the background level and further assessment is not justified. However, it is important to note that background concentrations in some cases may present a risk: for example emissions from motor vehicles can result in higher exposure to residents in a transport corridor. Residences adjacent to refuelling stations may be exposed to high hydrocarbon background. Naturally elevated background can result from highly mineralised geologic environments, resulting in enrichments of potentially toxic trace metals or from anthropogenic inputs such as atmospheric deposition in highly industrialised areas that are often found in major Australian cities. In such cases, consultation with local environment regulators and health protection agencies would be appropriate.
Tier 1 screening criteria (including HILs and HSLs) should only be used where there has been adequate characterisation of a site (that is, appropriate representative sampling has been carried out). At the very least, the maximum and the 95% UCL should be compared to relevant Tier 1 screening criteria. Where sufficient data is available it may be appropriate for the arithmetic mean (AM) to be compared to the relevant Tier 1 criteria. However, the implications of localised elevated values should also be considered. The results should also meet the following criteria:
No single summary statistic will fully characterise a site and a range of relevant statistical measurements should be considered in the data evaluation process and iterative development of the CSM (refer to Schedule B2, Section 4). It is preferable to examine a range of summary statistics including the contaminant range, median, geometric mean and geometric SD and 95% UCL as well as the AM and AM SD. Further information is provided in Schedule B2.
The end-point of the Tier 1 screening is the selection of the contaminants of potential concern that require further assessment. If the Tier 1 screening assessment concludes that there are no contaminants with plausible pathways to exposed populations, then the assessment is complete.
Further assessment after Tier 1 screening may comprise either additional assessment at Tier 2 (that is, risk assessment as described by the remainder of this guidance) or, alternatively, additional data collection may be required.
A weight-of-evidence approach, whereby the consistency of data from more than one line of evidence is considered, is recommended. For example, soil contamination at a site should also be evaluated by examining data from other media such as air, groundwater and surface water. This data should also be analysed in order for it to be used in the appropriate exposure scenario at a site. There is less guidance available on the characteristics of such data and indicators of its sufficiency. An initial review of these other types of data is required to determine if the sampling and analysis methods used were appropriate and if the detection limits achieved were appropriate given the risk-based screening criteria.
Groundwater data being used to assess human exposure may consider a reasonable maximum and relevant average at the site or off-site (as appropriate from the CSM). Where groundwater is to be extracted and used for any purpose, the suitability of groundwater data for the assessment of average and maximum exposures should be on a site-specific basis.
If air data or soil vapour data is available for the site, then the use of that data needs to be considered within the context of the CSM and the activities at the site that may affect the presence of the chemicals in the air. Consideration of both a reasonable maximum and a relevant average case should be developed where possible.
Data generated from chemical analyses may fall below the limit of detection (LOD) or limit of reporting (LOR) for the analytical procedure. Although substitution methods (such as replacing with the LOD/2) are reported widely in the literature for analysing data with non-detects, these approaches result in bias of summary statistics calculated from the adjusted data set. Where less than about 15% of the relevant data set comprises non-detects, substituting half the detection limit may be satisfactory (US EPA 2006). More detailed adjustments may be appropriate where greater than 15% of results are below detection. Further information can be found in Schedule B2 and US EPA (2006 and 2007a).
Exposure assessment involves the estimation of the magnitude, frequency, extent and duration of exposures to contaminants. In the data evaluation stage, the CSM is refined to produce an understanding of the source(s), pathway(s) and exposed population(s) that require assessment at Tier 2. In the exposure assessment stage, the CSM is used to generate a numerical representation of the exposure pathways that can be modelled quantitatively; these are the model input values. The input values are then used to model exposure point concentrations and estimate intakes of contaminants by the exposed populations.
To promote consistency and transparency in making exposure assessment assumptions, enHealth has established a framework for exposure assessment, set out in enHealth (2012a). This section follows the framework, adding details specific to contaminated land assessment.
Exposure assessment modelling methods have largely been developed for use in contaminated land risk assessment by the US EPA, and in the following section there are many references to US EPA guidance documents. These documents are extensive, well referenced and provide model algorithms and guidance on their use. Adoption of the modelling methodology in this Schedule does not imply endorsement of the US guidance; rather the US methods should be adapted to meet Australian policy objectives and environmental circumstances.
In the following sections, the derivation of model input values generally using point estimates (that is, single value estimates) is discussed. A more complete discussion of the use of probability distributions as input values (for example, the Monte Carlo method) is provided in Section 2.4.4. There may be circumstances where the use of probabilistic methods is appropriate in Tier 2 assessments, and it is not the intention of this Schedule to discourage the use of probabilistic models where their use can be beneficial to the outcome of the assessment.
The key stages of exposure assessment, as applied to contaminated land risk assessment, are to:
Stages 1 to 4 comprise the translation of the CSM into modelling terms as described. Stages 5 and 6 are achieved using a quantitative health risk assessment model. Stage 7 is a test of the model.
The data evaluation stage should provide a good understanding of the source. Consideration needs to be given as to how the contaminant concentration will be applied in a Tier 2 human health risk assessment; that is, what values will be used as the input concentrations representative of site conditions. Note that, depending on the complexity of the site, there may be more than one ‘source’ requiring further assessment. Note also that the source may be in soil, water, non-aqueous phase liquid or vapour. Sources in different physical forms generally should be assessed separately and require separate input values. The depth of different sources also requires consideration. For example, contamination may be present in soil at depth during site investigations, but be moved to the surface of the site during construction.
There are a number of options for choosing the value to use as an input concentration. The most appropriate method will depend on the data set, and different methods may be required for different source areas or contaminants, since these may show very different distributions. If a series of data over time is available, consideration of trends will be needed; this is particularly important for groundwater sources. Some commonly used approaches are described herein; however, more sophisticated statistical methods may be used if the data set is suitable. Whatever approach is used to define the source input value, it should be clearly explained and justified.
Maximum observed contaminant concentration. This generally provides a conservative assessment because if estimated risks from the maximum concentrations are not of concern, then the site should be suitable for use under the CSM considered. Maximum concentration is often suitable for groundwater sources where trends are poorly defined. However, a maximum concentration may not be representative of the source as a whole and may result in an overestimation or underestimation of risk if the data is extremely limited.
Mean concentration. The mean contaminant concentration can be a suitable input concentration provided that it can be shown that it adequately represents the source being considered. It is important that small areas of high concentrations or hotspots are not ignored by averaging with lower values from other parts of the site. The mean value may be more representative of the source as a whole than the maximum, and may provide a better estimation of the actual concentration that a population would be exposed to over a period of time.
95% upper confidence limit (UCL) of the arithmetic mean contaminant concentration. This provides a 95% confidence level that the true population mean will be less than, or equal to, this value. The 95% UCL is a useful mechanism to account for uncertainty in whether the data set is large enough for the mean to provide a reliable measure of central tendency. Note that small data sets result in higher 95% UCLs. Further guidance on the use of 95% UCLs can be found in NSW DEC (1995), US EPA (2006) and US EPA (2007a).
Monte Carlo (or other probabilistic) techniques. Refer to Section 2.4.4.
Source input values will normally be soil, groundwater or soil vapour data. In more detailed assessments, more specific sources may be defined, such as dust, bore water or ambient air.
Considerations in this section are also relevant to contaminant source input values derived using fate and transport or other exposure point estimation methods. These are described elsewhere in this Schedule.
This stage involves describing the physical environment in terms of the input values that will be used to represent exposure pathways in the model. The scenario being modelled should clearly relate to the existing or proposed land use for which decisions on contamination are required. Depending on the pathways modelled, a number of variables will need to be defined, for example, soil type, soil properties, depth to groundwater, soil and vapour sources, climactic variables and building characteristics and dimensions. Schedule B7 provides a complete list of the exposure pathway variables used to derive the HILs, together with justification of the parameter values selected.
Where the CSM is similar to that used to derive the HILs, exposure pathway input parameters used to conduct a site-specific risk assessment should consider those adopted in the derivation of the HILs, with further consideration of site-specific factors (where relevant). Where the CSM is different from the HIL scenarios, further consideration of site-specific input parameters and variables may be warranted.
Each variable used in a model should be clearly referenced and justified. Some commercially available models do not permit amendment of all the variables listed and the user has to rely on the default values supplied with the software. Where this is the case, it should be demonstrated that the model defaults are applicable to the site. It should be appreciated that models developed for the use in other countries may incorporate assumptions that are not justified in the Australian environment (science policy and physical environment). Reference should be made to the physical setting assumptions outlined in Schedule B7 for guidance on values likely to be suitable for Australian sites. Site-specific data from site investigations should be used wherever possible.
The purpose of this part of the risk assessment is to determine the characteristics and behaviour of the critical exposed populations. Exposed populations may relate to the current or proposed future use of the site, and it should be made clear which land use assumptions are being made. Exposed populations may be located at some distance from the site, with pathways involving transport via groundwater, surface water or wind. Exposed populations may also be linked to the site via the food chain, for example, consumers of fish, meat or agricultural products that may be affected by site contaminants.
The physical characteristics and behaviour patterns representative of the exposed population should be selected for modelling. This is essentially a common sense exercise, which does not normally require any specific assessment methodology or data.
The main considerations are described below.
Physical characteristics selection of representative values for physical aspects such as age, life expectancy, body weight and respiration rate need to be applied. It is not expected that risk assessors will be generally required to generate these assumptions (refer to those listed in B7 and/or enHealth (2012a and 2012b) where relevant)—considerable uncertainty is involved and variations in assumptions can have a significant impact on the risk assessment outcome. Exposed population physical characteristics should be sourced from applicable Australian guidance on exposure assessment (i.e. enHealth 2012b). Schedule B7 provides the values that have been selected to derive the HILs, which are primarily sourced from enHealth (2012a and 2012b).
Exposed population behaviour exposure assessment requires the development of a model behaviour pattern that is judged to represent an exposed population. Some data on Australian behaviour patterns is available (for example, see EPHC 2004, enHealth 2012b). Important considerations in contaminated land risk assessment include factors such as the distribution of hours spent indoors and outdoors, amount of time spent in the location where exposure is predicted, level of physical activity, the nature of work or leisure activities, and the exposure duration. In selecting values to represent exposed population behaviour, it is important to consider the following:
It is recommended that in designing the exposure assessment, worst-case scenarios (particularly those where many ‘high-end‘ assumptions are compounded) should generally be avoided. The sensitivity and uncertainty of the assumptions adopted in the exposure assessment should be considered.
An exposure point concentration is the estimate of the concentration of the source contaminant in the medium that the population is exposed to, at the location where exposure is predicted to occur.
It is preferred that, where possible, exposure concentrations are derived from direct measurements in the relevant media (soil, bore water, indoor air, fruit and vegetables and eggs).
However, under some circumstances it is not practical to measure concentrations directly, and in these cases exposure point concentrations are typically estimated using computer models.
The most commonly used exposure point estimation methods are:
These methods are described herein, with the exception of groundwater fate and transport modelling for which guidance is presented in Schedule B2. It should be noted that the level of uncertainty associated with the use of any model for the purpose of estimating exposure concentrations should be carefully considered and discussed.
Volatile substances are those that are capable of changing from liquid to vapour phase (that is, volatilising) under ambient conditions. While there are a few definitions of what may be considered of significance with respect to volatilisation, a volatile substance can be defined as having a Henry’s law constant of greater than or equal to 10-5 atm/m3/mol and its vapour pressure greater than 1 mm Hg at room temperature (NJDEP 2005). In addition to these measures, a substance should be assessed as volatile if its saturated vapour concentration results in exposure concentrations that are a risk to the exposed population. Some chemicals with low Henry’s law constants, or low vapour pressures, are so toxic that even a small amount that moves into the vapour phase could be enough to contribute to a risk. Hence both measures of volatility and toxicity need to be considered.
Vapours may arise primarily from three processes (refer to US EPA (2004a) for additional detail and equations):
To assess exposure to volatiles, it is necessary to estimate the concentration of the vapour in the air that the exposed population breathes. The most direct approach to the quantification of these exposures is to use direct measurements of indoor or ambient air. Vapours in ambient air are relatively easy to sample; however, the collection and interpretation of this data can be difficult.
An indoor air sampling program may be expensive if many samples over a reasonably long period are needed to get representative results. In homes and workplaces, gaining access can be difficult and may lead to unnecessary concern on the part of the occupants.
Depending on the volatile compounds considered, ambient air results may be difficult to interpret since many other sources in addition to the site soil and groundwater can be present. For these reasons, ambient air measurements are not generally available. When they are available they may be unsuitable as a means to assess risks associated with soil and groundwater source alone, because of the inability to distinguish whether a soil- or groundwater-derived component is present or not. Where affected by background sources, the collection of indoor or ambient air measurements may not be considered the most appropriate approach.
Where direct measurements are not available, indoor and outdoor ambient air contaminant concentrations can also be predicted by modelling from measured soil vapour concentrations. Soil vapour measurement is the preferred route in most situations where a vapour issue (from a subsurface source) is considered likely to exist. However where soil vapour measurements are used in risk assessments, the relevance of the data with respect to the CSM and the data quality need to be evaluated prior to use. Guidance on the development and use of vapour CSMs is provided in Schedule B2 and references there-in.
In the absence of measured soil vapour concentrations, it is also possible to model the generation of vapour from soil, groundwater and non-aqueous phase liquids. This procedure adds another level of uncertainty to the process, and may lead to inaccurate results.
The uncertainties associated with the use of a model for these purposes should be well understood and discussed in relation to the nature of the volatile contaminants assessed.
Where unresolved uncertainties or unacceptable risks are predicted by modelling vapour concentrations, direct measurement of soil vapour and/or exposure concentrations indoors and outdoors should be obtained.
Additional information on developing a multiple-lines-of-evidence approach to assessing vapour risk can be found in Schedule B2.
The direct measurement of indoor air concentrations may be appropriate where practical and where the data can be adequately interpreted. When assessing impacts from contaminated land it is important that background influences on indoor air concentrations are identified and characterised.
There are many circumstances where the measurement of indoor air concentrations is not the preferred method of assessing exposure from a subsurface contamination source. The information should be used in conjunction with other measurements such as sub-slab soil vapour, soil vapour at depth and outdoor air.
If direct measurements of indoor air (soil vapour and outdoor air) are conducted, sufficient numbers of samples to address temporal and spatial variability need to be collected.
Alternatively, indoor air concentrations can be modelled (estimated) using an attenuation factor (refer to US EPA 2012b for a range of values that could be considered), a model such as the Johnson and Ettinger (1991) model, or another appropriate (justified) model. The Johnson and Ettinger (1991) model is a one-dimensional ‘heuristic’ analytical solution to model advective and diffusive vapour transport into indoor spaces. It provides an estimated attenuation coefficient that relates the vapour concentration in the indoor space to the soil vapour concentration at the source of contamination (US EPA 2004a). A vapour attenuation factor, ‘’, is calculated, which is the ratio of the concentration of a chemical vapour in an indoor scenario relative to that measured in the soil. This model has been updated and modified since 1991 (Abreu & Johnson 2005, 2006) and is also described in Davis et al. (2004, 2009a). Inputs to the model include chemical properties of the contaminant, saturated and unsaturated zone soil properties, and structural properties of the building (US EPA 2004a).
The Johnson and Ettinger model as described by US EPA (2004a) is the most commonly used model for estimating vapour concentrations in indoor air and has been used in the derivation of the Health Screening Levels (HSLs) for petroleum hydrocarbons. The US EPA model provides additional functionality permitting the estimation of soil vapour concentrations from soil, groundwater and phase separated liquid. It is provided at: http://epa.gov/oswer/riskassessment/airmodel/johnson_ettinger.htm
There are a number of ways in which this vapour model can be manipulated to improve the confidence in the outcomes from the model; however, confidence in any model output without corresponding data for the purpose of validation is not high. Additional guidance is provided in Davis et al. (2004, 2009a).
It is noted that the Johnson and Ettinger model (as described by US EPA (2004a) and other similar vapour intrusion models do not adequately address vapour risk issues where there are preferential vapour migration pathways connecting a vapour source with the building, where the building structure extends into a saturated contaminated zone (that is, into the groundwater table) or where biodegradation is of significance (see Section 4.4.5). These issues should be addressed on a site-specific basis using more suitable (justified) techniques.
The direct measurement of outdoor air concentrations is appropriate where practical, taking care with any data collected to minimise background influences.
Alternatively, outdoor air concentrations can be estimated using models such as the Jury et al. (1983) model. The Jury et al. model calculates the maximum flux of contaminant vapours from an infinite soil contaminant source via vapour phase diffusion. Chemical movement to the atmosphere is modelled via volatilisation loss through a stagnant air boundary layer at the soil surface, making it appropriate for use in an outdoor exposure setting. The Jury et al. (1983) model is widely accepted as an appropriate methodology for vapour modelling into outdoor air and has been applied by regulatory agencies in the United Kingdom (EA 2009) and United States (US EPA 1996) in the development of Tier 1 soil investigation levels. The Jury et al. model is also recommended for estimating outdoor vapour concentrations in the Standard guide for risk-based corrective action (ASTM E1739–95 (2010)).
The modelling of outdoor air exposures also needs to account for vapour dispersion, between the soil surface and breathing zone of potentially exposed populations. This can be done using an outdoor box model which may predict ambient vapour concentrations on the downwind edge of the area source at the breathing zone height, as described by ASTM (E1739–95 (2010)). Alternatively, vapour dispersion in a well-mixed box may be estimated using calculated air dispersion factors, as described in US EPA (1996). Either method is considered appropriate.
The Johnson and Ettinger model is constructed as both a steady-state solution to vapour transport (infinite or non-diminishing source) and as a quasi-steady-state solution (finite or diminishing source) for soil contamination. A finite source model was not provided for groundwater in US EPA (2004) since groundwater migration reduces the certainty of concentration attenuation.
In situations where the soil or non-aqueous phase liquid source of dissolved phase volatile groundwater contamination is no longer present, dissolved phase concentrations should diminish or attenuate as the contaminants volatilise (or biodegrade). Dissolved phase contamination therefore becomes ‘finite’.
In circumstances where relatively small amounts of contaminants are present in the source zone, the infinite source assumption can give rise to a physically impossible output when a long period of time is modelled. Assessments should consider whether sufficient source (considered on a site-specific basis and may address aspects that include mass, extent and potential for biodegradation) exists to support the volatilisation modelled for the time period under consideration.
The finite source model can be used for site-specific risk assessment, provided that field evidence for the finite nature of the source is presented.
In common with experience in the United States (US EPA 2012c), there are two classes of volatile substances that together account for a large percentage of sites affected by soil and groundwater contamination in Australia:
The principal behaviour difference between petroleum hydrocarbons and chlorinated solvent vapours in the subsurface is that petroleum hydrocarbons readily biodegrade under aerobic (oxygenated) conditions whereas chlorinated solvents typically biodegrade much more slowly and under anaerobic conditions (Davis et al. 2009b, US EPA 2012a, 2012b and 2012c).
Important factors that influence aerobic biodegradation in the unsaturated zone include source concentration, total oxygen demand (the oxygen required to biodegrade the organic contaminants as well as any soil organic matter), the distance between the source and the building and soil type (US EPA 2012a). For some petroleum vapour concentrations there may be sufficient separation distance between a persistent vapour source and a building foundation, referred to in some published literature as ‘exclusion distance’, that attenuation of biodegrading petroleum chemicals is essentially complete, as the supply of oxygen is non-limiting. Consideration of exclusion distances may be useful in the assessment of the significance of the vapour pathway for petroleum hydrocarbons. Further information is provided in Schedule B2 (Section 9).
However, aerobic biodegradation may be limited by the supply of oxygen such as may occur under large areas of hardstand or where soil organic matter consumes the available oxygen. Aerobic biodegradation is also not applicable to chlorinated solvents (except vinyl chloride where evidence suggests good biodegradation in the presence of oxygen) and other volatile contaminants. Chlorinated hydrocarbons, however, are known to biodegrade in highly reducing environments. Incomplete degradation of chlorinated solvents can produce toxic degradation products such as vinyl chloride and this should be considered where relevant.
Davis et al. (2009b) recommend a process for incorporating biodegradation into vapour assessment of petroleum hydrocarbons where there is sufficient evidence to justify its use (and where site-specific biodegradation data is not available). This approach may be applied in the context of the HSLs for petroleum hydrocarbons (see Schedule B1 Section 2.4.9). It is noted that the measurement of oxygen in the soil profile can be difficult and care should be taken when using this data to support biodegradation.
Approaches to assessing and modelling biodegradation of petroleum hydrocarbon vapours are developing rapidly. Useful methods are likely to be developed and will come into general use. For example the American Petroleum Institute (API) in 2009 released Biovapor, a model permitting simulation of biodegradation (including the consideration of the presence of a slab) on a site-specific basis. It can be accessed and downloaded at www.api.org/ehs/groundwater/vapor/index.cfm.
While currently it is not possible to recommend Biovapor as an appropriate method for use in Australia, it is not the intent of this guidance to restrict development of new approaches and techniques for improved assessment. Therefore, where new methods can be justifiably applied, their use may be considered.
Non-aqueous phase occurs when the sorbed phase, aqueous phase, and vapour phase of a chemical have reached saturation in soil. Concentrations above this saturation limit (Csat) for all of the specified chemicals of a mixture result in a non-aqueous phase liquid or solid (US EPA 2000).
At contaminant concentration less than Csat, the equilibrium vapour concentration at the contaminant source is proportional to the soil concentration, according to the vapour modelling equation presented by Johnson and Ettinger (US EPA 2004a). When a non-aqueous phase is present however, the vapour concentration at the contaminant source is independent of the soil concentration but proportional to the mole fraction of the individual component of the non-aqueous phase mixture, according to Raoult’s Law (US EPA 2000). It is noted that the calculation of Csat relies on laboratory derived parameters and hence there may be some variability in calculated Csat values and site-specific values associated with the observed presence of non-aqueous phase liquids.
Raoult’s Law states that ‘the vapour pressure of each chemical component in an ideal solution is dependent on the vapour pressure of the individual component and the mole fraction of the component present in the solution’.
Therefore, as the number of components in a solution increases, the individual vapour pressures decrease as the mole fraction of each component decreases with each additional component.
In order to calculate the mole fraction for mixtures, or a solution of compounds, it is necessary to know the concentrations of the individual components comprising the non-aqueous phase liquid. This cannot be achieved by estimating the proportion of components in non-aqueous phase liquid from dissolved phase results. A sample of the free phase liquid should be collected and analysed if practicable.
Mole fraction = Number of moles of compound
Total number of moles
Number of moles = Concentration of compound in solution
Compound molecular weight
The saturation vapour concentration can therefore be calculated by:
Csat | = | X ρ MW |
|
| R T |
where:
Csat = Saturation vapour concentration (g/cm3)
X = Mole fraction of chemical in product
= Vapour pressure of the chemical (mm Hg)
MW = Molecular weight of compound (g/mole)
R = Molar gas constant (62, 361 mm Hg - cm3/mole - k)
T = Absolute temperature (293 K for ambient conditions)
Where contamination is present in surface soils or there is potential for near-surface contamination to be brought to surface during construction activities, exposure to particulates (dust) requires consideration.
The concentration of particulates relevant for different exposure scenarios can be determined using either a dust concentration factor or a soil particulate emission factor (PEF). The PEF approach is presented in the soil screening guidance (US EPA 1996) and supplemental soil screening guidance (US EPA 2002a). The methodology uses a fixed conservative soil particulate release rate combined with a box model to determine dust concentrations in air. The PEF assumes loosely packed surface soil so that a relatively large concentration of dust is entrained in air. The entrained soil particles are considered to mix in the ambient air breathing zone directly above the soil source. The calculation of a PEF using the US EPA equations may not be applicable to Australian conditions and hence suitability of the equation should be considered prior to use. In addition, the model does not address exposures associated with dust that is generated from dry, exposed soil, generated during active use of a site (for example, during use of dry sporting fields) or mechanically generated (such as during vehicle movements). Dust generated during these scenarios may be better estimated using a dust concentration (loading) relevant to the area and nature of activity assessed or alternatively, exposure may be assessed by conducting air quality monitoring.
Ingestion of contaminated food is a potential pathway for humans to be exposed to hazardous chemicals and, in some site assessments, may be a significant pathway requiring quantitative risk assessment. Guidance on how to calculate the mean daily (contaminant) intake from ingestion of contaminated food is provided in the following hierarchy of sources:
Patterns in food consumption vary between individuals and groups of individuals and therefore the likelihood exists that some groups of people will have a different diet from the population as a whole (Cross & Taylor 1996). Australian national food consumption data, including percentage of each food type or group consumed on a daily basis is available in enHealth (2012b) and has been compiled from a number of dietary surveys.
EnHealth (2012a) strongly recommends that Australian dietary survey data is used in exposure assessments, as overseas diets are likely to be of less relevance, though the algorithms from overseas guidance are applicable to Australia. When using the dietary survey data, it is important to consider the limitations of the survey conducted and the site-specific situation being addressed (particularly in relation to food consumption patterns) within a multiple-lines-of-evidence approach. Additional guidance, including contaminant intake algorithms for different produce that are also applicable to the Australian context, is available in the US EPA exposure factors handbook (US EPA 2011) and EA (2006).
When assessing contaminant intake from food consumption, it is important to consider not only the percentage of a particular food group in the diet, but also the percentage of the food group that could potentially have been grown/reared in a contaminated environment.
In circumstances where home-grown fruit and vegetable consumption is likely to be significant (for example, more than 10% of the diet), the consumption of garden vegetables grown in soil on a contaminated site is likely to represent the main potential transfer of soil contamination to adults and children. An assessment of exposure from this pathway depends on three critical factors: how much contamination is likely to be accumulated by garden vegetables from the surrounding soil, how much home-grown produce is likely to be consumed by those in the household, and how much contamination in food is absorbed by the human body (Paustenbach 2000).
Limited published information is available on the percentage of Australian households having domestic fruit and vegetable gardens, and on the percentage of home-grown fruit and vegetables consumed in comparison to those purchased. However, health risk assessments are site-specific and therefore this information may be obtained through discussion with the site owner/occupier at the CSM development stage. In the absence of site-specific information, Cross and Taylor (1996) provide a summary of information currently available.
Contaminant uptake behaviour varies markedly between plant species and for different contaminants. Ideally, the concentration of contaminants in home-grown vegetables and fruits should be measured directly on a site-specific basis, but this is often impractical in contaminated land assessments. In the absence of site-specific contaminant uptake data, the chemical concentrations in the edible portions of fruits and vegetables can be predicted from the soil-to-plant concentration factors (CFs), which describe the relationships between the concentrations of contaminants in the soil and plant contaminant concentrations, on a fresh weight basis.
A methodology to estimate contaminant intake from fruit and vegetables for home-grown vegetable intakes of up to 10% of the diet is provided in Schedule B7.
It is recommended that caution be employed in the evaluation of potential human health risks associated with ingestion of fruits and vegetables using generic predictions of plant uptake. Data is limited, and the methods for predicting plant uptake of contaminants from soil and groundwater data are not well validated, particularly for many organic substances. Further site-specific investigations are likely to be justified in situations where the consumption of home-grown produce is likely to constitute a significant portion (>10%) of the diet of a household or where commercial production of vegetables and/or fruit may occur.
The calculation of intake concentrations from the consumption of poultry, meat and fish is complicated and suffers from a number of uncertainties that limit the reliability of the assessment. In order to estimate contaminant concentrations in the potential food source using soil, groundwater and surface water concentrations, the following information is required as a minimum:
Data is scarce for each element and there is currently no generic methodology available for estimating contaminant levels in animals resulting from their exposure to contaminated soil or water.
If the exposure of farm livestock has already occurred, direct measurement of the contaminant levels in the meat would be the most practical means to estimate intakes of potentially exposed populations. Some studies have been conducted on fish and shellfish and equations for estimating contaminant concentrations in fish from water concentrations are available in ANZECC and ARMCANZ (2000) and Arnot and Gobas (2003). Additional algorithms on uptake in humans from consumption of contaminated fish are also provided in the ANZECC and ARMCANZ (2000) guidelines.
In the absence of directly measured contaminant concentrations in fish, the ANZECC and ARMCANZ method is recommended to estimate the likely concentration of contaminant uptake into fish tissues and subsequently the mean daily intake dose from human consumption. Implementing the ANZECC and ARMCANZ methodology involves:
Contaminant intake is estimated for each chemical and pathway separately. The recommended methodology generally follows US EPA (1989). There are two basic approaches that have been developed on the basis of the way in which chemicals cause toxicity:
For threshold chemicals, the estimated daily intake can be compared with a threshold toxicity reference value (TRV), which is a value representing a dose that will cause no adverse effect over a lifetime of exposure. Note that, as applied here, the TRV means any appropriate measure of tolerable daily intake, and includes doses derived by various bodies for different applications. Reference doses can be tolerable daily intakes (TDI), usually from drinking water guidelines (for example, Australian or WHO), or acceptable daily intakes (ADI) typically used in food and drug guidance, or US EPA reference doses (RfD).
Threshold TRVs are often available for ingestion and inhalation exposure routes, and occasionally for dermal exposure. Since the TRV for different exposure routes can be quite different, each TRV is only applied to the relevant pathway. Consequently, estimated intakes are only summed to the extent that they correspond to the same exposure route (that is, all ingestion pathways added, all inhalation pathways added, but ingestion intake not added to inhalation intake) when estimating risk by comparing intake to the TRV. The relative scarcity of TRVs for the dermal exposure route is usually accommodated by adding the dermal intake to the ingestion intake where a dermal TRV cannot be sourced. Once hazard/risk quotients and indices are calculated, summing of all pathways can and should be conducted as outlined in the risk characterisation discussion in Section 6.
For carcinogens that are considered as non-threshold chemicals (generally genotoxic carcinogens), intakes are estimated on a daily basis, multiplied by the exposure duration (ED) and then divided by the averaging time (AT). AT is typically a value representing a lifetime. This results in a daily intake, which is multiplied by a non-threshold TRV—such as a cancer slope factor (CSF) or unit risk factor (URF)—to produce a measure of excess lifetime cancer risk.
Doing the calculations in this fashion assumes that it is the daily average exposure over the whole lifetime that is relevant to the development of cancer.
Methodologies and algorithms for estimating ingestion intakes are available for the pathways listed below. Note that the data applied within these methods should be sourced from Australian publications such as enHealth (2012a and 2012b) as far as possible. Details of the data used to generate the HILs are given in Schedule B7 and in Friebel and Nadebaum (2011a) for the HSLs.
The equations available to estimate these intakes are generally of the form shown below and detailed in Table 1 for the direct soil ingestion pathway (US EPA 1989).
Intake (mg/kg-day) = CS x IRS x CF x FI x EF x ED
BW x AT
All currently available exposure models for contaminated land assessment use equations of this form, although there may be variations in detail. Values for the variables in Table 1 (and similar variables for other ingestion pathways) should be derived during the stages of exposure assessment described earlier in this section.
Table 1. Variables description for soil ingestion intake calculation
Variable | Units | Description |
CS | mg/kg | Concentration in soil |
IRS | mg soil/day | Soil ingestion rate |
CF | 10-6 kg/mg | Unit conversion factor |
FI | - | Fraction ingested from contaminated source |
EF | days/year | Exposure frequency |
ED | years | Exposure duration |
BW | kg | Body weight |
AT | days | Averaging time |
Dermal intakes can be estimated for the following pathways:
Dermal contact with vapour phase contaminants is not generally assessed since it is likely to be insignificant in comparison with other pathways (US EPA 1989).
Dermal absorbed dose or dermal intake is estimated using the concept of absorbed dose per event (US EPA 2004b). The overall absorbed dose depends on the number of events, the adherence factor (AF) and the fraction of contaminant absorbed (ABS).
It is noted that there are a number of uncertainties inherent in the estimation of both AF and ABS. US EPA (2004b) provides estimates of ABS for eleven substances or groups of substances and provides guidance on the treatment of uncertainty for the assessment of substances where specific ABS values are not available. AF is dependent on soil type, activity type and exposed population age, and values are provided for a range of circumstances in Exhibit C-2 in Appendix C of US EPA (2004b). Because soil type is so important to the value of AF, additional guidance is provided for sediment (although the method is the same). Wet soil and fine-grained soil have much higher values of AF than dryer and coarser soil.
The equation for dermal intakes is published as follows and detailed in Table 2.
Dermal intake (mg/kg-day) | = | DAevent x EF x EV x ED x SA |
|
| BW x AT |
For soil, DAevent = CS x CF x AF x ABS
For water, the calculation of DAevent is dependent on the exposure duration.
For short duration exposures (tevent ≤ t*): ![]()
For long duration exposures: 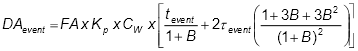
Table 2. Variables description for dermal contact intake calculation
Variable | Units | Description |
DAevent | mg/cm2-event | Dermal absorbed dose per event per unit exposed skin area |
EF | days/year | Exposure frequency |
EV | events/day | Event frequency |
ED | years | Exposure duration |
SA | cm2 | Skin surface area available for contact |
BW | kg | Body weight |
AT | days | Averaging time |
CS | mg/kg | Concentration in soil |
CW | mg/cm3 | Concentration in water |
CF | 10-6 kg/mg | Unit conversion factor |
AF | mg/cm2-event | Adherence factor of soil to skin |
ABS | - | Dermal absorption fraction (chemical-specific) |
FA | - | Fraction absorbed from water |
Kp | cm/hour | Dermal permeability coefficient of compound in water (chemical –specific) |
τevent | hours/event | Lag time per event (chemical-specific, refer to Appendix B of US EPA (2004b)) |
t* | hours | Time to reach steady state = 2.4 τevent |
tevent | hour/event | Event duration |
B | - | Ratio of the permeability coefficient of a compound through the stratum corneum relative to its permeability coefficient across the viable epidermis (refer to Equation A.1 in Appendix A of US EPA (2004b)) |
Inhalation intakes can be estimated for the following pathways:
The mechanism for deriving the vapour and dust concentration in air for the above pathways is described in an earlier section of this Schedule. The quantitative approach outlined in US EPA (1989) was developed before the US EPA issued the Inhalation Dosimetry Methodology (US EPA 1994). In this regard, US EPA (2009) updates the inhalation dosimetric approach to be compatible with the Inhalation Dosimetry Methodology, which represents the US EPA's current methodology for inhalation dosimetry and the derivation of inhalation toxicity reference values.
Risk Assessment Guidance (RAGS) Part F (US EPA 2009) recommends that when estimating risk via inhalation, risk assessors should use the concentration of the chemical in air as the exposure metric (e.g. mg/m3), rather than inhalation intake of a contaminant in air based on inhalation rate (IR) and body weight (BW) (e.g., mg/kg-day). The US EPA “recommends that the intake equation presented in RAGS, Part A (US EPA (1989), Exhibit 6-16) should no longer be used when evaluating risk from the inhalation pathway”. Hence for the quantification of exposures via inhalation of dust or vapours, it is appropriate that guidance available from US EPA (2009) is considered.
The equation for inhalation intakes of vapours and dust is published as follows and detailed in Table 3.
ECvapour (µg/m3) | = | CA x ET x B x EF x ED |
|
| AT |
ECdust (µg/m3) | = | CA x RF x ET x B x EF x ED |
|
| AT |
Table 3. Variables description for vapour inhalation intake calculation
Variable | Units | Description |
B | -- | Bioavailability |
ECvapour or ECdust | µg/m3 | Exposure concentration (a time-weighted average concentration) relevant to chemicals present as a vapour or dust |
CA | µg/m3 | Concentration in air (exposure point concentration which has been measured or modelled) |
RF | - | Lung retention factor relevant to the inhalation of dust and includes consideration of a deposition fraction and ciliary clearance |
ET | hours/day | Exposure time |
EF | days/year | Exposure frequency |
ED | years | Exposure duration |
AT | hours | Averaging time |
For threshold chemicals the estimated exposure concentration (EC) is compared to the inhalation specific toxicity reference value for assessment of chronic or subchronic risks. For non-threshold chemicals, excess cancer risk is calculated by multiplying the URF by the EC. Note that a method for the assessment of acute exposure risk is also provided by US EPA (2009), in which the CA is compared directly to the appropriate toxicity reference value. When sourcing inhalation toxicity reference values using the hierarchy of data sources listed in Table 4, the inhalation value may be an air quality guideline.
There is a substantial body of evidence that links environmental exposure to lead to uptake into the blood stream. The impact of lead on cognitive processes, especially of children, is also well understood. Several studies have been undertaken at Port Pirie in South Australia showing the relationships between maternal blood lead and pregnancy outcome and children’s abilities at various ages following environmental exposure to lead (e.g. Country Health SA 2007 at www.publications.health.sa.gov.au/envh). Studies suggest that an increase of 10 µg/dL of lead in blood (PbB) can lead to an IQ decrease of 1 to 5 points and recent studies show that there may be no lower threshold on the effect of lead in blood (ATSDR 2007).
A risk assessment technique has been developed to assess the uptake of lead into the blood, which effectively applies an a priori uptake factor to an estimated dose to estimate blood lead concentration. The US EPA integrated exposure uptake biokinetic model (IEUBK) for lead in children is widely known and used in this respect. In addition, the US EPA adult lead methodology may be an appropriate tool to assist in the assessment of adult lead exposures. Both of these tools are available at www.epa.gov/superfund/lead/products.htm.
This approach with the appropriate justifications is considered suitable at Tier 2 for assessing risks from lead.
The US EPA continues to develop a research-oriented biokinetic model, the all-ages lead model, which is designed to potentially replace the integrated uptake biokinetic model for lead in children and includes a full population age range (090 years) and updated uptake and biokinetic modules. This model is, however, still under peer review and has not been formally approved for application in contaminated land assessments. Information on the all-ages lead model is available at:
http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=139314.
In contaminated land health risk assessment, we are commonly estimating the intake of a chemical in soil, and comparing this to a tolerable daily intake or reference dose. The intake of contaminant is estimated from the intake of soil, using soil concentration data that nominally represents the ‘total’ concentration of the substance in soil. The toxic effect of a contaminant actually depends upon the uptake or absorbed dose of contaminant, that is, the amount that gets into the bloodstream after being ingested, inhaled or via skin contact. The fraction of a compound that is absorbed into the body (systemic dose) following exposure via all pathways (administered dose) is generically termed the ‘bioavailable fraction’.
More specifically:
The assessment of contaminant bioaccessibility may also be considered for estimating contaminant uptake. Bioaccessibility is related to the solubility of the contaminant in the gastrointestinal tract. More specifically, in the context of soil contamination, it is defined as the fraction of a contaminant in soil that is soluble in the relevant physiological milieu (usually the gastrointestinal tract) which is potentially available for absorption. This can be assessed by validated in vitro test systems.
TRVs used in risk assessment have been derived from animal experiments or from epidemiological data (e.g. arsenic). In most cases, TRVs are derived from the administered dose (i.e. intake of the pure substance), rather than the absorbed dose or the absorption of a substance from soil (note that this should be reviewed for all chemicals of concern to determine relevance) and hence account for the bioavailability of the pure substance. However, because they rarely, if ever, intrinsically account for the soil matrix, relative bioavailability is not accounted for.
It is usually not necessary to account for absolute bioavailability (as defined above) in the oral and inhalation pathways, because it may already be incorporated in the TRV. The derivation of the TRV should be understood, with reference to how the experimental dose was administered. In cases where doses have been injected, for example, there may be a case for introducing a factor to represent the bioavailability of the substance when administered orally or by inhalation.
In the oral and inhalation pathways, it should in theory always be reasonable to introduce a factor to allow for relative bioavailability, since this is almost never intrinsically part of the reference dose. Unfortunately, data is limited and so it is not appropriate to introduce this factor except where more generic relative bioavailability values are available (currently limited to arsenic and lead, however site-specific assessments are often required in addition to the use of generic values).
Relative bioavailability of contaminants in soil is complicated, highly variable and difficult to predict. This is because it depends strongly on the nature of the soil matrix (for example, soil type, age of soil, organic carbon, potential particle size, etc.) and on environmental conditions, particularly redox potential. HILs are derived using 100% relative bioavailability assumptions with the exception of lead and arsenic (refer to Schedule B7, Appendix A1). In site-specific assessments, however, it may be appropriate to refine this assumption.
A detailed review was carried out by Ng et al. (2009) as part of the review of the NEPM. It concluded that in vitro assays were appropriate as a surrogate method for estimating relative bioavailability for some contaminants (e.g. lead and arsenic), and could be used for Tier 2 assessments. Currently it is considered that there are no reliable in vitro methods for other contaminants though further research may provide adequate validation in the future.
In vivo methods are available for the resolution of contaminated land issues though these methods are not likely to be practical due to their expense.
There are a number of in vitro methods that may be considered as a surrogate measure of arsenic and lead relative bioavailability. These may include Relative Bioavailability Leaching Procedure (RBALP) (US EPA 2007b), the Solubility Bioavailability Research Consortium (SBRC) (Kelley et al. 2002) or the in vitro gastrointestinal method (IVG). However it is noted that the selection and use of any in vitro method should be conducted on a contaminant-specific basis where the availability, validity and limitations of available methods are considered at the time of the assessment.
There do not appear to be any validated analytical methods for estimating inhalation bioaccessibility, especially for particulates. For the assessment of vapours, given that these chemicals tend to cross the lung membranes fairly easily, assuming 100% relative bioavailability is appropriate.
In risk assessments, the dermal pathway has a well-established mechanism for considering absorption and relative bioavailability. The lack of dermal-specific TRVs means that a dermal dose is compared to the ingestion TRV. However the dermal dose represents an absorbed dose rather than applied dose (as is commonly the case in establishing ingestion TRVs). Hence it may be necessary to modify the ingestion TRV. This is commonly done by applying a gastrointestinal absorption factor (GAF) to the ingestion TRV, which modifies the TRV by a factor that addresses absorption of the chemical across the gastrointestinal tract in the critical toxicity study. There are limitations with this simplistic approach as it does not address metabolism in the gut wall or skin or first-pass effects associated with orally administered doses. In some cases it may be more appropriate to use a dermal specific TRV.
For soil-bound contaminants, there is little data on the influence of matrix on dermal absorption. A common approach to address this issue is to apply a dermal absorption fraction (ABS, described in Section 4.7.3) to modify the applied dose in soil to calculate the dermally absorbed dose. It represents the proportion of the contaminant in soil that is considered to be absorbed into the bloodstream through the skin.
Petroleum products have a high degree of variability in their physical properties and chemical compositions. Products such as gasoline, diesel, fuel oil and jet fuel each have their own chemical signatures and the composition of the same product can vary depending on where it was distilled and the source of its crude oil. This makes environmental assessment of these products difficult and an approach has been developed that can assess the broad and varying range of compounds in a uniform manner.
Internationally recognised publications on the composition and assessment of petroleum hydrocarbons are available from the TPH Criteria Working Group (TPHCWG 1997a, 1997b, 1998). These documents present criteria for breaking total petroleum hydrocarbons (TPH) down into aromatic and aliphatic fractions with associated data available on the physical chemistry and toxicity of each fraction. It is based on choosing a relevant substance in each fraction and assuming that all the chemicals that are included in that fraction have the same toxicity as the surrogate chemical.
The TPHCWG approach was developed to provide a consistent and transparent method for dealing with petroleum hydrocarbons in risk assessment. Application of the TPHCWG approach requires the assessment of carcinogenic indicator compounds (such as benzene and benzo(a)pyrene) in addition to the TPH fractions. The alternative to adopting the TPHCWG approach is either to assume that the risks from petroleum mixtures can be adequately assessed using indicator substances such as benzene and hexane, or to attempt to analyse for individual substances and assess each one separately.
The former approach is practical; however, it creates problems in determining transparent clean-up criteria for the bulk of the TPH, since absence of benzene and benzo(a)pyrene does not obviously ensure absence of risk. The latter approach would be rigorous, but given that there are thousands of compounds in TPH mixtures, it would not be practical. The TPHCWG method is therefore the recommended approach.
Analytical data for soil, groundwater and phase-separated hydrocarbons can be obtained on the aromatic-aliphatic composition of TPH compounds in terms of carbon numbers, and can be grouped into their corresponding TPHCWG fractions.
TPHCWG does not provide inhalation reference values for carbon numbers greater than C16 as these fractions are not considered to be volatile (refer to definition in Section 4.4.1). With respect to the inhalation pathway for TPH C16-C36 (aliphatic and aromatic fraction), TPHCWG (1998) states that:
‘there are no appropriate data available for the development of RfCs [inhalation reference concentrations] in this carbon range. Also, the development of an inhalation RfC from this fraction was determined to be inappropriate because the compounds in this carbon range are not volatile and inhalation will not be a relevant exposure pathway’.
Where the inhalation of dust is of importance in a risk assessment, it may be appropriate to use route extrapolation of oral TRVs for the assessment of TPH C16-C36 (aliphatic and aromatic fraction).
HSLs have been developed for benzene, toluene, ethyl benzene, xylenes, naphthalene, and petroleum hydrocarbon fractions (C6-C10, >C10-C16, >C16-C34 and >C34) in soil, soil vapour and groundwater for use where the key contaminants are petrol and/or diesel. The TPH HSLs were derived using TPHCWG aromatic and aliphatic fractions. Where other products (for example, aviation fuels, fuel oils, kerosene) are present, analysis of the aromatic and aliphatic fractions separately is necessary to determine the composition, since this can have a significant impact on the risk.
Where phase-separated hydrocarbon is present, a site-specific assessment of vapour risk including analysis of the aromatic and aliphatic fractions may be necessary (for further information see Section 4.4.6).
Toxicity assessment is typically divided into two activities:
These descriptors can have widely varying meanings, depending on the scope and purpose of the risk assessment. The sections herein describe the processes as they apply to assessing risks from contaminated land, with particular focus on site-specific decision-making.
Further and more general guidance on toxicity assessment is provided in enHealth (2012a).
Ideally, such assessments need to be undertaken by an appropriately qualified toxicologist. This is definitely required if any attempt is made to develop a TRV directly from toxicity data in the literature rather than use a TRV already developed by a government authority.
Toxicity assessment in contaminated land risk assessment is primarily a literature-based research exercise. For contaminants which have an HIL, the findings of the literature review undertaken are presented in Schedule B7. In many risk assessments, reference to the appropriate review in Schedule B7 will provide adequate information to inform the toxicity assessment. In cases where no HIL is presented, or where the risk assessor is aware that more recent information is available, the toxicity review should be compiled and reviewed by an appropriately qualified toxicologist from reliable peer-reviewed sources.
In principle, risk assessments would ideally be based on research that has been carried out, peer reviewed and recommended by Australian health authorities as appropriate for Australian circumstances. In practice, there is limited Australian-specific information available, and Australian health standards for air quality and drinking water (for example, NEPC 2004, NHMRC 2011) are also largely based on international data sources, in particular, WHO publications.
There are a number of readily available web-based authoritative sources of toxicity information, which are designed for the purpose of informing risk assessments. In general, published Australian data and the classification of carcinogens as reported by the International Agency for Research on Cancer (IARC) should be used in risk assessments when available, but other data may be used where appropriately justified.
Data sources listed in Table 4 are considered to provide information that is compliant with Australian requirements for setting public health standards. They should generally be considered in the order listed in Table 4; however, it should be noted that the reliability of the data used in a risk assessment should not be based solely on the position of its source in the table. The most important consideration is that the data is supported by sound science and contemporary risk assessment methodologies, and that a weight-of-evidence approach has been used to assess their worth. This means that risk assessors should not simply rely on ‘looking up numbers’ but should gain an appreciation of the currency of the source and the underlying science from which the numbers have been derived. Such an evaluation should be undertaken by an appropriately qualified toxicologist.
Table 4. Sources of information for toxicity assessment
Reference | Description |
National Health and Medical Research Council documents e.g. Australian drinking water guidelines (ADWGs) | NHMRC documents are a useful source of Australian-specific information. ADWGs in particular should be checked to ensure that Australian circumstances are understood. |
National Environmental Protection Council documents e.g. NEPM (Air Toxics), NEPM (Ambient Air Quality) and Air Quality Standard Setting Methodology | As above, provide useful information on Australian circumstances, although for a more limited range of contaminants than the ADWGs. NEPC publications can be accessed at www.scew.gov.au The fifth national workshop on the health risk assessment and management of contaminated sites was published by NEPC and is available at http://www.ephc.gov.au/taxonomy/term/60. |
Other Australian Government sources of toxicity criteria | The Australian Government publishes acceptable daily intakes and acute reference doses for agricultural and veterinary chemicals. These can be accessed at http://www.health.gov.au/internet/main/publishing.nsf/Content/ocs-adi-list.htm |
South Australia Health | South Australia Health published a series of workshops on risk assessment of contaminated land (1st to 4th National Workshops on the health risk assessment and management of contaminated sites, between 1991 and 1996). They can be accessed at http://www.publications.health.sa.gov.au/envh/. This website also provides a number of other publications that are useful for risk assessments. |
World Health Organization sources, for example, WHO air quality and drinking water quality guidelines, and Environmental Health Criteria series documents. Documents from the International Programme on Chemical Safety (IPCS) and Joint FAO/WHO Expert Committee on Food Additives (JECFA) are included in this group. | Australia is a party to the WHO process and has incorporated Australian material into a variety of Environmental Health Criteria documents. Australian drinking water guidelines (ADWGs) use WHO guidance as a primary resource. Most WHO documents are available on the web at www.inchem.org. |
International Agency for Research on Cancer (IARC) documents | IARC provides classification for carcinogens. The IARC classifications should be the primary source for carcinogen classification and should generally be used to determine whether chemicals will be considered as carcinogens for the purposes of the risk assessment. |
US Agency for Toxic Substances and Disease Registry (ATSDR) minimal risk levels and toxicological reviews | ATSDR information is often based on the same sources as the Integrated Risk Information System (IRIS) database, but is independent of the US EPA. Its toxicological reviews are available on the web. ATSDR provides minimal risk levels (MRLs) for threshold effects which can be used in the same way as RfDs. MRLs can be accessed at http://www.atsdr.cdc.gov/mrls/index.html. |
Other governmental sources of information on chemicals and risk assessment, e.g. NICNAS priority existing chemical reports, US EPA IRIS database, UK, Health Canada, Dutch and New Zealand guidance | Useful for general toxicity information, and may provide support data such as bioavailability/ bioaccessibility, estimates for background concentrations, methods for considering groups of chemicals, mixtures, cumulative effects etc. Should be accompanied by robust justification. Toxicity criteria should only be derived from these when none are available from any source listed above. |
Other sources of peer reviewed toxicity criteria including other US EPA sources such as the regional screening levels, the PPRTV or HEAST tables on which the regional screening values are based or state-based US agencies such as California EPA, OEHHA etc. | Other sources of toxicity criteria suitable for use in risk assessment include US EPA’s provisional peer reviewed toxicity values (PPRTV), California Environmental Protection Agency (Cal EPA) toxicity values, and the US EPA Superfund health effects assessment summary tables (HEAST). |
Peer reviewed journals | In general single source papers from journals should not be used to derive toxicity information for contaminated land risk assessment. They do not normally comply with the requirement for having carried out extensive literature review, and budgets for site-specific risk assessment will not normally support such review. Where information sourced directly from journals is used, robust justification should be provided. |
Physical and chemical data are necessary inputs to the exposure modelling process, and also provide useful information on fate and transport of the contaminant. This data is generally experimentally derived, and some values may vary considerably between sources. The source of physical and chemical data should always be quoted in risk assessments, and the effects of uncertainty considered. As an aid to consistency and transparency in Australian risk assessment, the following sources, in order, are recommended for the selection of physical and chemical data. These sources are those used to derive the HILs see Schedule B7.
Hazard identification is defined by enHealth (2012a), following Health Canada (1999), as the process of determining:
In contaminated land health risk assessment, these will generally be determined with reference to the data sources listed in Table 4. The health effects of a substance are likely to have been researched using a number of methods and the data sources adopt a weight–of-evidence approach in recommending which research to rely on. The reviews provided by the data sources are carried out by expert panels and the level of expertise and effort expended in scrutinising the primary sources of information cannot generally be reproduced in a site-specific risk assessment. A brief explanation of the methods used to derive information on health effects is given here. The reader is referred to enHealth (2012a) for more detail.
Information on health effects is usually generated by studies using animals (in vivo studies), studies involving people (epidemiological studies) or laboratory experiments using cells or tissue rather than live animals (in vitro studies). Relevant and reliable data on people is available for only a very few chemicals, and therefore most health effect information is derived by extrapolation from animal and laboratory experiments. This produces considerable uncertainty, since animals and people will not necessarily have similar responses to a chemical substance.
Further uncertainty in contaminated land health risk assessment is introduced by the fact that toxicological research usually focuses on evaluating a specific substance, and does not account for the complexities introduced by the substance’s presence in soil or in mixtures of substances.
Health effects can be broadly separated into acute and chronic effects. The distinction between acute and chronic exposure risks relates to the duration of exposure and timing for the onset of any health effects. Acute health effects occur within minutes, hours or days of a relatively short period of exposure, whilst chronic health effects occur as a result of prolonged or repeated exposures over many days, months or years and symptoms may not be immediately apparent.
Acute toxicity information on chemicals is widely available because it is used for the classification of manufactured chemicals for supply. Studies of acute effects using animal experiments may produce information on oral, dermal and inhalation toxicity, skin and eye irritation and skin sensitisation.
Standard protocols are available, such as OECD test guidelines (OECD 1998), resulting in a high degree of standardisation of available information. Acute reference doses are also available for some substances from the Australian Government (for example, pesticides – Table 4) and from the US EPA’s IRIS database. Another source of guidelines covering acute effects is the US NOAA public exposure guidelines which can be found at:
http://response.restoration.noaa.gov/topic_subtopic_entry.php?RECORD_KEY%28entry_subtopic_topic%29=entry_id,subtopic_id,topic_id&entry_id(entry_subtopic_topic)=659&subtopic_id(entry_subtopic_topic)=24&topic_id(entry_subtopic_topic)=1.
The soil HILs, and most contaminated land risk assessments, focus on chronic effects. This is because in most circumstances soil contamination capable of causing acute health effects would be self-evidently unacceptable. However, acute effects can be important during remediation works (for example, inhalation of vapours resulting in dizziness) and should not be ignored in hazard identification.
There can also be potential for physical hazards, for example, fire, explosion, and subsurface gas accumulation leading to asphyxiating atmospheres. These are managed by techniques beyond the scope of this Schedule though the hazard identification process should acknowledge them.
Chronic threshold effects cover all kinds of chronic toxicity other than cancer as well as those compounds that exhibit ‘threshold’ toxicity as described for non-genotoxic carcinogens. This may include sub-chronic effects (medium term, for example, less than 10% of a lifetime), effects on reproduction, or development of the foetus.
When assessing hazards, attention should be given to the derivation of threshold reference values and the relevance of that derivation to the environmental contaminants under consideration. Toxicity assessment should seek to identify hazards that are relevant to the form of the contaminant in soil, water or air and should not assume ‘worst-case’ toxicity on the basis of substances or exposure pathways that are implausible, for example, where the effect was based on an occupational study looking at the inhalation of metal fumes. Since the metal in soil would not be capable of causing fumes, this hazard would be inapplicable and could be reasonably discounted.
Hazard identification for these compounds should detail the following:
Chemicals may cause cancer in a wide variety of ways, termed modes of action. There is flexibility in defining modes of action, which can be described at almost any level of complexity, reflecting the extent of chemical-specific information available and the needs of the risk assessment (Clewell 2005; Lambert & Lipscomb 2007). It is now known that several modes of carcinogenic action exist, including mutagenicity, mitogenesis, inhibition of cell death, cytotoxicity with reparative cell proliferation, and immune suppression (US EPA 2005a; Butterworth 2007). Although it represents a simplification, for the purposes of risk assessment, cancer-causing chemicals (carcinogens) are generally divided into two types, genotoxic and non-genotoxic.
Genotoxic carcinogens are defined as chemicals for which there is adequate evidence of the potential to interact with and/or modify the functions of genetic material and which has the ability to induce tumours via a mechanism involving direct damage to DNA (Butterworth 1990; IARC 2006). For genotoxic carcinogens, it is assumed that no level of exposure is entirely safe and even at extremely low levels some damage to the genetic material may increase the chance of developing cancer.
This is known as a non-threshold (or linear) dose-response relationship and the application of a threshold — based on ‘no observable adverse effect level’ (NOAEL) — is not considered appropriate.
Non-genotoxic carcinogens are chemicals that induce tumours via a mechanism which does not involve direct damage to genetic material (IARC 2006). For non-genotoxic carcinogens, it is assumed that a threshold dose can be determined below which no toxic or carcinogenic effects are seen (that is, a non-linear dose-response relationship can be established). A common approach for determining a safe dose for chemicals that exhibit a threshold or non-linear dose-response relationship is the selection of a NOAEL (or other point of departure) from relevant animal or human studies.
Complexity is added by the uncertainty in whether some chemicals are carcinogenic or not, leading to difficulty for the risk assessor in deciding which approach to consider. Different sources of information may provide different views on whether a substance is a carcinogen or not. The US EPA in particular regards a wide range of chemicals as potential carcinogens and this view is not always shared by health authorities in other countries. The IARC classification is summarised in Table 5.
In some circumstances, a chemical may have a carcinogenic classification but lack toxicity criteria to assess the cancer risk. In these circumstances, it is recommended that the chemical be assessed using a threshold approach (that is, ignoring the cancer risk) in modelling. The possibility of cancer effects should be identified and evaluated, even though a numerical expression of risk cannot be derived.
Table 5. IARC classification for carcinogens
Group | Description |
1 | Agent1 is carcinogenic to humans |
2A | Probable human carcinogen, an agent for which there is limited evidence of carcinogenicity in humans and sufficient evidence of carcinogenicity in experimental animals |
2B | Possible human carcinogen, an agent for which there is limited evidence of carcinogenicity in humans and less than sufficient evidence of carcinogenicity in experimental animals |
3 | Not classifiable as to their carcinogenicity to humans |
4 | Probably not carcinogenic to humans |
1 — IARC defines an agent as ’specific chemical groups, groups of related chemicals, complex mixtures, occupational or environmental exposures, cultural or behavioural practices, biological organisms and physical agents (IARC 2006). | |
There may also be circumstances where the data relating to the classification of a substance as a carcinogen is not relevant to soil contamination. This would apply, for example, where the cancer classification was based on an occupational study where inhalation of metal fumes had been found to cause cancer. Since the metal in soil would not be capable of causing fumes, this hazard would be inapplicable and could be reasonably discounted.
Hazard identification for carcinogens should detail the following:
The doseresponse assessment looks at establishing a quantitative relationship between the exposure to a chemical and the effect realised. For the purposes of contaminated land health risk assessment, doseresponse assessment comprises selection of suitable reference values from the authoritative data sources listed in this Schedule.
The term toxicity reference value (TRV) refers to measures of tolerable dose that are derived by expert panels on behalf of government or international bodies responsible for public health guidelines or standards. The TRVs recommended have been developed for use in risk assessment or in setting public health guidelines or standards. They incorporate allowances for uncertainty in the studies upon which they are based and generally also accommodate (where possible) safety factors to provide for particularly sensitive groups in the population. The information provided to support the TRVs invariably provides a description of the process followed.
It is recognised that these approaches have a number of limitations and that a more unified approach would be sensible. Concern has also been expressed in the international risk assessment community that the current approach both underemphasises non-cancer effects, and provides an unrealistic ‘bright line’ divide between possible harm and safety (NRC 2008). Future developments in doseresponse assessment are likely to move towards a unified approach covering all chemicals and perhaps a single mechanism to express risk.
Currently, the threshold and non-threshold approaches still dominate risk assessment practice, and the TRVs associated with each are described below.
The US EPA’s IRIS database describes the toxicity criteria for ‘threshold’ substances as follows:
‘Oral reference doses and inhalation reference concentrations (RfDs and RfCs, respectively) for effects known or assumed to be produced through a nonlinear (possibly threshold) mode of action. In most instances, RfDs and RfCs are developed for the non-carcinogenic effects of substances.’
Where a carcinogen is assessed on the basis of a threshold doseresponse relationship, the threshold TRVs adopted need to consider both carcinogenic and non-carcinogenic end-points.
For threshold chemicals, TRVs reflect a measure of tolerable daily exposure. The threshold is typically selected from a point of departure in the dose-response curve below which adverse effects are not observed (NOAEL) and includes a range of safety (or uncertainty) factors. There are a number of terms that are used by different agencies to define a threshold TRV. Most commonly these include an ADI (acceptable daily intake), TDI (tolerable daily intake), TC (tolerable concentration in air), RfD (reference dose), RfC (reference concentration), MRL (minimal risk level) and REL (reference exposure level), as noted in Table 6.
In contaminated land health risk assessment, the estimated exposures from oral (ingestion), dermal and inhalation routes are compared to the corresponding TRV. This process is described in the risk characterisation section of this Schedule.
The US EPA’s IRIS database describes the TRVs for ‘non-threshold’ substances that it presents as follows:
‘Descriptors that characterise the weight of evidence for human carcinogenicity, oral slope factors, and oral and inhalation unit risks for carcinogenic effects. Where a nonlinear mode of action is established, RfD and RfC values may be used.’
For non-threshold chemicals, TRVs reflect a cancer risk value commonly referred to as a cancer slope factor (CSF) or unit risk factor (URF).
Table 6. Threshold toxicity reference values
Toxicity criterion | Units | Meaning |
Acceptable daily intake ADI | mg/kg-day | The daily intake of a substance that, during a lifetime, appears to be without appreciable risk, on the basis of all the facts known at the time (WHO 1994). The term ADI is generally used for chemicals such as pesticides that are deliberately used on food or crops. ADI is very similar conceptually to RfD; the different terminology arises from the measure having been defined by a different body. |
Tolerable daily intake TDI | mg/kg-day | An estimate of the intake of a substance that can occur over a lifetime without appreciable health risk (WHO 1994). TDI is generally used when a chemical is a food or environmental contaminant. Like ADI, TDI is conceptually similar to RfD. |
Reference dose RfD | mg/kg-day | An estimate (with uncertainty spanning perhaps an order of magnitude) of a daily oral intake of a substance by the human population (including sensitive populations) that is likely to be without an appreciable risk of deleterious effects during a lifetime. It can be derived from a NOAEL, LOAEL or benchmark dose, with uncertainty factors generally applied to reflect limitations of the data used (IRIS). Generally used in non-cancer health assessments. |
Reference concentration RfC | mg/m3 | An estimate (with uncertainty spanning perhaps an order of magnitude) of a continuous inhalation intake of a substance by the human population (including sensitive populations) that is likely to be without an appreciable risk of deleterious effects during a lifetime. It can be derived from a NOAEL, LOAEL or benchmark concentration, with uncertainty factors generally applied to reflect limitations of the data used (IRIS). Generally used in non-cancer health assessments. |
CSFs are typically calculated for the assessment of carcinogenic end-points for genotoxic carcinogens (that is, non-threshold compounds). A slope factor is a plausible upper-bound estimate of the probability of a response per unit of intake of a chemical over a lifetime. The slope factor is used in risk assessments to estimate an upper-bound lifetime probability of an individual developing cancer as a result of exposure to a particular level of a potential carcinogen (US EPA 1989). This approach is also known as the ‘linear’ approach which implies a proportional (linear) relationship between risk and dose at low doses. CSFs assume that there is no level of exposure to carcinogenic chemicals that does not pose a finite probability, however small, of generating a carcinogenic response.
A URF is an expression of carcinogenic potency in concentration terms, such as probability of cancer per 1 μg/L of drinking water or probability of cancer per 1 μg/m3 in air. Generally, the drinking water URF is derived by converting a CSF from units of mg/kg-day to units of μg/L, and an inhalation URF is developed directly from a dose-response analysis using equivalent human concentration already expressed in units of μg/m3 (US EPA 2005a). Derivation of a URF often assumes a standard intake rate (for example, inhalation of 20 m3 of air per day or ingestion of 2 L of water per day) and body weight (for example, 70 kg). When a theoretical upper-bound cancer risk estimate is calculated using a URF instead of a CSF, it is often termed the unit risk.
Unit risk factors can be converted to slope factors as follows:
CSFinh = URinh x 1000 x 70/20
where
CSFinh = inhalation cancer slope factor (mg/kg-day)-1
URFinh = inhalation unit risk factor (reported as per µg/m3), which is converted to a CSF assuming 70 kg body weight and 20 m3/day inhalation volume.
Benchmark doses are typically calculated for carcinogenic substances that are considered to be non-genotoxic (that is, threshold compounds). A benchmark dose is defined as the dose that corresponds to a specified change in adverse response compared to the response in untreated animals (Crump 1995).
The dose is associated with a given incidence (for example, 1%, 5% or 10% incidence) of effect, the benchmark risk, based on the best fitting doseresponse curve in the region of the doseresponse relationship where biologically observable data are available (Filipsson et al. 2003; enHealth 2012a). Although the benchmark dose has mainly been used for risk assessment of non-cancer end-points, this approach can also be applied for cancer end-points. When selecting benchmark doses, recent Australian guidance recommends adopting a 10% incidence (NEPC 2011).
Increasingly, the benchmark dose approach for cancer risk assessment is being adopted globally in recognition of the identified difficulties and uncertainties associated with the low-dose extrapolation method (that is, generation of cancer slope factors). A benchmark dose is applied in the same way as a threshold TRV.
There is often a choice to be made in selection of doseresponse data for carcinogens. Recommendations are summarised below.
For a more detailed treatment of the methodologies applied to assessing cancer risks, refer to the review of cancer risk methodologies in the ASC NEPM Toolbox at http://www.scew.gov.au/archive/site-contamination/asc-nepm.html.
It will not normally be necessary for risk assessors to derive toxicity reference values for use in site-specific risk assessment. This should only be considered when criteria have not been developed by sources listed in this Schedule and should only be undertaken by a suitably qualified toxicologist.
In cases where the potential site contaminants (that is, known or suspected to be present in the site soil) have no TRVs, but are not detected above the lowest detection limit that can be achieved using available analytical methodology, it is considered acceptable to assume that no additional assessment is necessary. Note that ‘available’ in this context would include internationally available. The extent of effort expended in procuring a suitable analysis should be proportional to the probability of the contaminant’s presence and severity of suspected toxic effects.
Where derivation of new TRVs is required, an extensive and robust literature review undertaken by appropriately qualified toxicologists is expected. The procedure for establishing toxicity reference values is set out in enHealth (2012a) and should be followed. Note that the process requires a high degree of expert judgement.
Special consideration has been advocated by the US EPA for assessing risks associated with early-life exposure to mutagenic carcinogens (refer to enHealth 2012a and US EPA 2005a for further information). US legislation also mandates the application of an additional 10x safety/uncertainty factor in the derivation of an RfD for pesticides where studies indicate developmental neurotoxicity or other toxic effects that could be associated with early-life susceptibility.
With respect to non-threshold reference values, the US EPA guidance recommends that individual compounds are assessed to determine whether there is a mutagenic mode of action and whether the potential for early life-time susceptibility should be considered. Where relevant, the following can be applied:
This process should be considered for individual contaminants where there is clear evidence of a mutagenic mode of action.
A chemical ‘species’ is the specific form of an element defined by its oxidation (valency) state and/or complex or molecular structure. Some of these structural levels are more important for risk assessment than others. In particular, valency state and inorganic and covalent organometallic speciation are of great importance in determining the toxicity of metals and metalloids (WHO 2006). Elements occur in soil in either the solid phase or in the soil solution. In the solid phase, ions can be bound to soil components by means of ion exchange or surface complexes or they can occur as minerals or be co-precipitated as minerals in soil. In the soil solution they can occur as free ions or complexes.
Standard chemical analysis provides a measure of ‘total’ metal in soil or water, expressed as a concentration of the elemental form. This is not particularly informative as a means of assessing how toxic the soil or water could be.
Further difficulty is introduced because toxicological research rarely focuses on the metal species most likely to be present in soil. Typically, the focus is on the most toxic forms and on those that are of particular health concern as a result of their presence in food, consumer products or in the workplace. This means that the available TRVs for metals and metal compounds may significantly overestimate the toxicity of the metals in soil and water.
Some examples of variations in toxicity with chemical species include the following:
The chemical species of a metal can affect its toxicokinetics by influencing its absorption, distribution, biotransformation and elimination. It is therefore important that risk assessments should consider the species rather than the elemental constituent in order to create meaningful data.
Some typical elemental species in soil are summarised in Table 7.
The speciation of metals in soil and water can be determined to some extent by chemical analysis; however, this is expensive and the data obtainable is limited. Assumptions regarding speciation will normally have to be made using available understanding of the site conditions and site history. It is recommended that where analytical information is not available, risk assessments considering metals should account for metal species using a reasonable worst-case assumption regarding the presence of toxic forms of the metal. TRVs should then be selected for relevance to the assumption presented.
The reasonable worst-case assumption should be derived from the following:
Other indicators such as iron content and the presence of other contaminants that may influence metal speciation may also be useful.
Table 7. Elemental species that may be present and influence the toxicity of elements in soil (WHO 2006)
Element | Aerobic soil | Anaerobic soil |
Arsenic | Ca3(AsO4)2, Mg3(AsO4)2, As2O5 | As2S3 |
Cadmium | Cd(OH)2, CdCO3 | CdS |
Chromium | Cr(OH)3 (low to neutral pH) | Cr(OH)3 |
Lead | PbO, PbCO3, Pb3(CO3)(OH)2 | PbS |
Mercury | HgCl2, HgO, Hg(OH)2 | HgS |
Nickel | NiO, NiCO3, Ni(OH)2 | NiS |
The reasonable worst-case assumption should be to assume that the metal in soil is the most toxic form that could be present given the understanding of the conditions. Forms that are not stable under environmental conditions, or that would require implausible soil processes to produce, should be discounted.
An alternative means of determining elemental speciation of aqueous solutions is through geochemical equilibrium speciation modelling. Examples of such models include MINTEQA2 (Allison et al. 1991) and PHREEQC (Parkhurst & Appelo 1999). Where reliable analytical methods cannot be found to conduct relevant elemental speciation analyses, geochemical modelling methods can be used to predict species in solution and phases that are likely to precipitate from solution. The accuracy of such methods is largely dependent on the accuracy of the input data. Such geochemical modelling methods are applicable to both soil solutions and aqueous environments.
Typical input data required to run geochemical models includes basic geochemical field parameters (pH, electrical conductivity, redox potential, dissolved oxygen and temperature), major ion chemistry (sodium, potassium, calcium, magnesium, ammonium, chloride, carbonate, bicarbonate, sulfate, nitrate, nitrite, phosphate), iron, manganese and the trace metal chemistry of the soil solution. The essential data needed to perform a speciation calculation is the temperature, pH, and the concentration of elements and element valency states.
Where there is reasonable evidence to suggest that a metal contaminant may be present as a species that is less toxic than the toxicity assumed by the HIL, analytical or modelling methods may be useful in characterising the geochemistry of the particular environment. In this way, a more accurate prediction of the toxicity of the metal contaminant can be made.
The final step of the health risk assessment process is risk characterisation. In this step, information from the data collection, exposure and toxicity assessments is summarised and integrated into quantitative and qualitative expressions of risk. Risk characterisation conveys the risk assessor’s judgement as to the nature and existence of (or lack of) human health risks, in an informative and useful manner for decision-makers.
Risk characterisation can be considered a three step process:
The aim of risk characterisation is to:
There are a number of principles which form the basis for the risk characterisation process, including the following:
Risk estimation combines the estimated intakes calculated in the exposure assessment with the TRVs (threshold and non-threshold where relevant) from the toxicity assessment to produce numerical indices of likely health effect. The risk estimation methodology differs for threshold and non-threshold compounds due to the different modes of chemical effect.
For threshold compounds, the intake for each exposure pathway is divided by the appropriate threshold TRV (allowing for intakes from other sources where relevant) to produce a simple ratio, termed a hazard quotient (HQ) or risk quotient (RQ). The HQs for all exposure pathways for each contaminant can be summed to produce a total hazard index (HI) or risk index (RI).
Hazard Quotient (HQ) | = | Intake (mg/kg-day) |
|
| Threshold TRV (mg/kg-day) |
Hazard Index (HI) | = | Σ Hazard Quotients |
The HQs for all exposure pathways for all contaminants should be summed to produce a total HI, unless evidence is available to show that this is not appropriate. When summing these HQs, the following should be taken into consideration:
Where non-threshold TRVs are adopted (that is, assuming a linear low-dose relationship), risks are estimated as the incremental probability of an individual developing cancer over a lifetime as a result of exposure to the carcinogen. The estimated intake for each exposure pathway and non-threshold TRV are multiplied to produce pathway-specific estimates of increased lifetime cancer risks (ILCR).
However, for those carcinogens where appropriate benchmark dose data are available, the risk estimation method outlined above for threshold compounds applies.
ILCR | = | Intake (mg/kg-day) x TRV(mg/kg-day)-1 |
ILCR | = | exposure concentration (mg/m3) x TRV(mg/m3)-1 |
ILCR estimates from all pathways and all contaminants should be summed to produce a total increased lifetime cancer risk, unless evidence is available that suggests that this is not appropriate. When combining ILCR estimates, the US EPA (1989) identifies several limitations which may be considered.
These include:
In practice, it will often be the case that there is insufficient information to make a well-informed decision as to whether it is reasonable or not to sum ILCRs across either pathways or contaminants (see also Section 6.5 of this Schedule). It is recommended that a precautionary approach (set out below) should be followed under most circumstances. Where more information is available, a decision to assess contaminants or pathways as independent and non-additive should be supported with reference to the toxicology of the contaminants concerned by appropriately qualified toxicologists.
The threshold HI assumes that there is a level of exposure below which it is unlikely for sensitive populations to experience health effects. If the exposure level does not exceed the threshold (that is, HI less than 1), then it is reasonable to conclude that no adverse health effects are likely to be realised. If the exposure level exceeds the threshold (that is, HI greater than 1) then further consideration is necessary. An HI greater than 1 does not automatically imply that an unacceptable risk is present but does indicate that further action(s) (investigation or risk management) is warranted. The HILs were developed on the basis of an HI of 1.
It is generally considered that the greater the HI, the greater the level of concern. HI ratios should not be interpreted as statistical probabilities; for example, an HI of 0.001 does not imply that there is a one in one thousand chance of the effect occurring (US EPA 1989).
Defining ‘negligible risk’ is a complex and potentially controversial issue. In debating this issue, consideration should also be given to the fact that people are routinely exposed to many naturally occurring carcinogens as a part of day-to-day living. Further, some people willingly expose themselves to carcinogens, for example, by smoking tobacco. However, people have the right to not be exposed to high risk agents artificially introduced into their environment. Furthermore, since the development of cancer is a step-by-step, cumulative process and people are exposed to many cancer-causing agents, the risk of any additional exposure should be negligible or set at a level that does not increase the existing risk by anything other than a negligible amount.
Historically, the life-time risk value of one in a 1,000,000 (often abbreviated as 1x10-6 or 10-6) was considered an insignificant risk and used as a maximum value for defining permissible environmental concentrations.
The US EPA has set a lifetime cancer risk goal of 1 in 1,000,000 for the regulation of individual genotoxic chemicals. However, in determining an acceptable cancer risk, EPA determined a safe emissions level, and then added a margin of safety in view of uncertainties in scientific knowledge. The US EPA adopted a general policy that a lifetime cancer risk of 1 in 10,000 for the most exposed person may constitute acceptable risk and that a margin of safety should be added to reduce the risk for the greatest possible number of persons to an individual lifetime risk no higher than 1 in 1,000,000 (NRC 1994).
The adoption of 1 in 1,000,000 as a criterion for assessing excess lifetime cancer risk was originally specified by the US Food and Drug Administration (FDA) in the 1970s to identify food additives that did not require further assessment. The criterion was an arbitrary decision and was not based on any scientific rationale. The criterion was also subsequently adopted by other US and international agencies without any rigorous debate (Kelly 1991).
The World Health Organization (WHO) has recognised that the setting of environmental standards for air and water contaminants is the responsibility of national agencies. WHO generally provides guideline values for genotoxic carcinogens corresponding to lifetime risks of 1 in 10,000, 1 in 100,000 and 1 in 1,000,000 from which national agencies can select a value to be used in their guidelines (WHO 2000). By basing its guideline values on risks between 1 in 10,000 and 1 in 1,000,000, WHO is implying that it considers that acceptable risk lies somewhere in the range 1 in 10,000 and 1 in 1,000,000. WHO also recognises that the acceptability of risk depends on scientific data, social, economic and political factors, and the perceived benefits arising from circumstances that might be associated with exposure to the carcinogen e.g. the health benefits of disinfecting drinking water, are greater than the risks that might be posed by potentially carcinogenic disinfection by-products.
In Australia there is no universally accepted criterion for acceptable excess lifetime cancer risk. Lower incremental lifetime cancer risk values (1x10-6) are commonly adopted in guidance that addresses population-wide exposures, such as the Australian Drinking Water Guidelines (NHMRC 2011), which has adopted a lifetime risk level of 1 in 1,000,000 for genotoxic carcinogens.
NSW EPA considers an excess lifetime cancer risk of less than 1 in 1,000,000 as acceptable and cancer risks greater than 1 in 10,000 as unacceptable (NSW DEC 2005). In between these two limits, proponents should demonstrate best practice for development applications to be approved.
In Victoria, for planning purposes, the Environmental Protection Agency uses an excess lifetime cancer risk of 1 in 100,000 for individual chemicals and cumulative excess lifetime cancer risk. However their intervention levels for chemicals in regional air sheds are based on an excess lifetime cancer risk of 1 in 100,000.
In Western Australia, the Department of Health (WA DoH 2009) has developed screening levels for asbestos that are based on a range of lifetime risks of 1 in 1,000,000 to 1 in 100,000.
The cancer risk criteria used by various international agencies are summarised in Table 8. The values range between 1 in 1,000,000 and 1 in 10,000.
Table 8. International Non-Threshold Risk Criteria Adopted as Acceptable
Organisation | Acceptable risk level | Context | Comments |
WHO | 1:100,000 | WHO Guidelines for Drinking Water Quality (WHO 2011) | Concentrations representing excess lifetime cancer risks of 10-4, 10-5 and 10-6 risk are presented, and the recommended guideline value is associated with 10-5 cancer risk. WHO (2011) accepts that this is a conservative recommendation, which ‘almost certainly overestimates the true risk’. WHO (2011) considers that ‘there is some (theoretical) risk at any level of exposure’ to carcinogens. |
WHO | Unit risk estimate (i.e. risk per 1 µg/m3) presented | WHO Air Quality Guidelines (WHO 2000, 2010) | WHO consider that the decision on the acceptability of a risk should be made by national authorities within the frame work of risk management. Similar to the WHO Drinking Water Guidelines (WHO 2011), concentrations in air associated with an excess cancer risk of 10-4, 10-5 and 10-6 are given. |
US EPA | 1:1,000,000 | US EPA (1996) Soil Screening Levels | EPA (1996a) believes that ‘… setting a 10-6 risk level for individual chemicals and pathways will generally lead to cumulative risks within the risk range (10-4 to 10-6) for the combinations of chemicals typically found at Superfund sites.’ |
Netherlands | 1:10,000 | Technical evaluation of the Intervention Values for Soil/sediment and Groundwater (RIVM 2001). | For genotoxic carcinogens, the acceptable excess lifetime cancer risk was set at 1 per 10,000 individuals. For non-genotoxic carcinogens, the MPR does not result in any adverse health effects during lifetime exposure (70 years) (RIVM 2001). |
United Kingdom | 1:100,000 | EA (2009) | UK generally prefers not to use quantitative expressions of acceptable risk and does not base policy specifically on them. 1 in 100,000 is the risk level used when the UK judges that a linear low dose extrapolation is the most appropriate basis for its Index Dose. |
Canada | 1:100,000 | Health Canada (2004) | This value was recommended given the conservative margin associated with slope factors and the negligible impact of a 1:100,000 incremental risk level for contaminated site exposures. |
When using non-threshold TRV as doseresponse criteria, the recommended acceptable incremental lifetime risk of developing cancer arising from exposure to single or multiple carcinogens is 1 in 100,000 (10-5) consistent with enHealth (2012a).
Contaminated land studies frequently involve assessing the health risks associated with soil where a number of different chemical contaminants are present. In contrast, toxicological studies usually assess the effects of a single chemical. The risk assessor faces a difficulty in determining whether the effects of the mixture might be additive, greater than additive (synergistic) or less (antagonistic) (Priestly 2009). It is possible that such effects are not important at the low doses common in environmental exposure, leading to the concept of an ‘interaction threshold’ below which the effects of mixtures are insignificant (Hamm et al. 2005). Additive effects are being found to be more common than synergism.
Despite the limitations in the data, risk assessors have been incorporating additivity into their assessments for some time. This is done by adding all risks from all chemicals together to get total risk for a site and also by adding risks from all pathways. There are likely to be situations where the chemicals cause quite different effects by quite different mechanisms and so it is possible that summing risks in this way can overestimate risks in such situations. However, most sites have a mix of chemicals that are similar because they have arisen due to the particular activities on a site.
Priestly (2009) has reviewed all the available approaches to the risk evaluation of mixtures including summing of risk quotients as already described, which are summarised below:
Hazard quotient approach uses the ratio of the estimated exposure to the measure of acceptable exposure (the hazard quotient) for each component of the mixture and adds them to produce a hazard index which is the expression of the likely acceptability of the mix. This approach is widely used in Australian risk assessment. It is recommended for the assessment of petroleum hydrocarbons by the England and Wales Environment Agency (EA 2005).
The approach suffers from a fundamental limitation, which is the inherent assumption that the components summed have a common mode of action, or at least a common end-point. Where this is not the case, the components are theoretically toxicologically independent.
This HQ approach is recommended as a screening tool for most Tier 2 risk assessments. It is particularly useful for the assessment of petroleum hydrocarbon mixtures, and other mixtures where the assumption that substances are likely to have similar toxicological effects can be justified.
Where the HI for the mixture is less than unity (HI<1), a conclusion that exposure is likely to be within acceptable bounds may be made. When HI>1, it does not necessarily indicate that a site poses an unacceptable risk but it does indicate that either further consideration should be applied, or risk management actions should be recommended. An HI >1 does not necessarily indicate unacceptable risk.
Further consideration would normally include assessment of the modes of action that the mixture components might exhibit. This Schedule provides guidance on how to apply the HQ approach.
Summation of non-threshold risk estimates (ICLRs) applies similar logic to the HQ approach. It is common to assess risk from genotoxic carcinogens in terms of a numerical estimate of increased probability of developing cancer over a lifetime. It is possible to sum these risks from components of a mixture to develop a combined estimate of total risk. The approach suffers from exactly the same limitation as the HQ approach, in that it assumes a similar mode of action or end-point, which may be unjustified.
This approach is recommended as a screening approach for all genotoxic carcinogens in a mixture. Where the sum exceeds the acceptable risk, the components should be assessed in more detail to look at whether the mode of action or end point justifies summing the risk. Where modes of action and/or end-points are clearly different, carcinogens should not be summed.
Assessment of representative mixtures by direct in vivo or in vitro experiments. This approach is clearly limited by the number of variations in relative concentration that could be tested, and also by other complexities of environmental contamination such as weathering and degradation. It is not likely that this approach will be widely applicable to contaminated land health risk assessment.
Toxicity equivalence factor (TEF) approach in which the potential effects of a group of similar substances are estimated relative to a single member of the group. The components of the mixture are assumed to contribute to the toxicity in a similar way, and their relative effect is calculated in proportion to their concentration in the mixture by adjustment using a relative potency factor. The approach has limitations in that the assumption of a similar mode of action may be unjustified in a variety of ways. The key limitation in a practical sense is that it is only available where a well-established set of relative potency factors exists for the components of the mixture. Currently its use is limited to carcinogenic PAHs, dioxins, PCBs and some endocrine-disrupting chemicals. This approach is recommended for carcinogenic PAHs, and guidance on how to apply it is provided in the toxicity profile for benzo(a)pyrene in Schedule B7 Appendix A2.
Component elimination or simplification approach may be used in circumstances where it is not reasonable to assume a common mode of action for components in a mixture. In this case, the components are assumed to be toxicologically independent. The outcome of this approach is to assume that the overall risk is no greater than the risk posed by the riskiest component of the mixture. This method should be applied where it can be shown that adding HQs or ILCRs is not reasonable.
Uncertainty in health risk assessment is the lack of knowledge about the correct value such as a specific exposure measure or estimate (enHealth 2012a). Uncertainty is distinguished from variability, which refers to true differences in attributes due to diversity or heterogeneity. Variability cannot be reduced by further measurement or study, although it can be better characterised (NRC 2008).
Both uncertainty and variability contribute to uncertainty in the estimation of risk and should be adequately assessed in a risk assessment. Such consideration needs to be done transparently so that all users of a risk assessment can understand the approach taken.
An analysis of the uncertainty in the risk assessment is important because:
Uncertainty analysis is generally a qualitative process though in some cases it can be semi-quantitative or quantitative.
The first step should be a consideration of the CSM and what aspects of that model are uncertain and how that uncertainty has been accounted for.
The second most important part of the uncertainty assessment is an evaluation of the uncertainty and variability in the site characterisation data. Site characterisation data will always be limited by time, site constraints and budgets. However, the risk estimates based on even quite limited data can be fit for purpose if the exposure concentrations are a long way below (or above) toxicity reference values, which indicate that the risks are either very low or very high. Decision-making based on such uncertain but quite clear results is straightforward. Where risks are close to or slightly above unacceptable (the ’grey‘ zone), the issue of the uncertainty and variability in the site characterisation data becomes much more important and so the uncertainty assessment needs to be more detailed.
When assessing risks, uncertainty can arise from missing or incomplete information, be incorporated into the scientific theory affecting the ability of a model to make predictions, and result from uncertainty affecting a particular parameter, for example, sampling errors. Such uncertainty has the potential to cumulatively overestimate or underestimate risk during an assessment. An assessment of uncertainty is a part of the health risk assessment process and consequently needs to be addressed for each step of the risk assessment and for its cumulative effect from all of the steps.
There are three broad types of uncertainty (US EPA 1992):
NRC (2008) provides a detailed evaluation of the techniques currently provided for in US EPA guidance and concludes that although a number of usable methodologies are provided, it is unclear what level of detail is required to capture and communicate key uncertainties. A further comment is that quantitative methods suffer from the difficulty in sensibly quantifying all uncertainties, and that the apparent precision of quantitative analysis for some uncertainties may distract attention from other, possibly equally important but unquantifiable, uncertainties.
In most health risk assessments for contaminated land projects, it is unlikely that quantitative uncertainty analysis (for example, US EPA 2001) will provide value given the effort required to undertake it. A clear qualitative analysis is considered sufficient in most cases to provide the communication of the effects of uncertainty that is necessary.
Further discussion and guidance regarding uncertainty is provided in enHealth (2012a). A useful example of an uncertainty analysis table is also provided in enHealth (2012a). NRC (2008) and WHO (2008) provide useful guidance on the principles to be adopted for uncertainty analysis; these have been adapted for specific relevance to contaminated land risk assessment herein:
The combination of uncertainty in the scientific data and assumptions (the ’inputs‘) and inability to validate assessment results directly or to isolate and evaluate the impact of a resulting decision (the ’outputs‘) creates a situation in which decision-makers, the scientific community, the public, industry and other stakeholders have little choice but to rely on the overall quality of the many processes used in the conduct of risk assessment to provide some assurance that the assessment is aligned with societal goals (NRC 2008).
Sensitivity analysis provides a quantitative estimate of the effect of uncertainty and/or variability in the input parameters on the results of the risk assessment.
Sensitivity analysis should be undertaken when a risk assessment is conducted using a deterministic exposure model. While a single value should be entered for each parameter in a deterministic model, it is unlikely that reasonable inputs for each parameter can be limited to a single value. This may be due to uncertainty (based on an absence of site-specific data, or site measurements, we may not know where the ‘true’ value lies) and/or variability (the ‘true’ value may vary across the site or over time due to variations in site geology laterally or with depth, or due to changes in site conditions over time). As such, a range of reasonable values will be defined as appropriate for a given input parameter.
Sensitivity analysis is the process of changing one variable within a defined range while leaving the others constant and determining the effect on the output. The procedure involves fixing each uncertain quantity, one at a time, at its credible lower-bound and then its upper bound (holding all other at their medians), and then computing the outcomes for each combination of values (US EPA 1992). It can be used to test the effects of both uncertainty and variability in input values.
Sensitivity analyses can be used to identify important input variables (or groups of variables) and develop bounds on the distribution of exposure or risk. A sensitivity analysis can also estimate the range of exposures or risk that result from combinations of minimum and maximum values for some parameters and mid-range values for others (US EPA 1989). Effort may then be directed to the collection of additional data for these important variables; as additional data is collected, the uncertainty in the ‘true’ value is reduced, and it may be possible to define a smaller range for a given parameter. The uncertainty in the results of the risk assessment may therefore be reduced.
All risk assessments where conclusions are derived using modelling should incorporate a sensitivity analysis and describe the variability in the model outputs generated by plausible variation in the inputs. Note that some input variables may be connected and unable to vary independently. Monte Carlo models, where inputs are described by probability distribution functions, provide probability distribution function outputs. The Monte Carlo method reduces the requirement for sensitivity analysis but may not eliminate it, depending on the model used.
Detailed guidance on risk communication and management is provided in Schedule B8 and the risk assessor needs to be aware of how the risk assessment will be used, as this is an essential step following the conclusion of a risk assessment.
Risk communication is the process of informing people about potential hazards to their person, property, or community. From the risk management perspective, the purpose of risk communication is to help affected communities understand the processes of risk assessment and management, to form scientifically valid perceptions of the likely hazards, and in some cases to inform decisions about how risk should be managed. There should be a clearly defined functional separation between risk assessment and risk management. In the US, many stakeholders believe that the current process for developing and applying risk assessments lacks credibility and transparency (NRC 2008). Although there does not appear to be specific research on this issue related to contaminated land risk assessment in Australia, the same may well be true.
The investigation, management and remediation of contaminated sites may give rise to a range of community concerns. These may be based on actual or perceived environmental risks and loss of amenity or nuisance. When planning a communication strategy, the factors relevant to the timing and extent of consultation should be identified. The extent of communication will vary, but should include all stakeholders in the vicinity of the site who may be physically affected by the site assessment or remediation or by loss of amenity or nuisance, as well as those who may not be physically affected but have concerns about the contamination (the broader community).
There are a number of underlying principles that should be considered in risk communication. These have been set out in various publications, including Core Values for the Practice of Public Participation (IAP2 2000), and propose that the community be provided with information and the opportunity to input to decisions that may affect their wellbeing. The community should also be advised about the proposed plan for risk communication. Planning for risk communication should therefore commence during the site investigation stage.
The communication plan should be flexible and identify and manage new information as it becomes available and provide ongoing dialogue with the community.
Guidance on risk communication is provided in Schedule B8, and by enHealth (2012a), as well as by state regulators (for example, WA DEC 2006) and international agencies (US EPA 2007c).
One of the key objectives of risk assessment is usually to support a decision about what to do about the contamination present on a site. For effective risk management, it is important that the potential risk management actions are considered at the planning stage (that is, issues identification). Failure to adequately plan is likely to result in a risk assessment that does not fully meet the needs of the risk manager and other stakeholders.
Risk management decisions are often taken by a group of stakeholders (for example, landowner, project managers, legal advisors and regulators), few of whom are expert in risk assessment. It is important that the results of risk assessment, including the consideration of uncertainty, are presented in a way that can be understood by non-specialists.
One of the key considerations in risk management is the extent to which remediation is needed in order to adequately mitigate the risk. Poor understanding of the risk assessment process and the inherent uncertainties can lead to lack of confidence on the part of stakeholders, often resulting in a decision to remediate to a higher standard than necessary (for example to the HILs).
Making a risk management decision requires informed consideration of the risks in the context of the site and broader stakeholder issues that may include practicality and community acceptability, cost/benefit, time scales for remediation and technical feasibility.
Abreu, L & Johnson, P 2005, ‘Effect of vapour source-building separation and building construction on soil vapour intrusion as studied with a three-dimensional numerical model’, Environmental Science and Technology, vol. 39, no. 12, pp. 4550–4561.
Abreu, L & Johnson, P 2006, ‘Simulating the effect of anaerobic biodegradation on soil vapour intrusion into buildings: influence of degradation rate, source concentration and depth’, Environmental Science and Technology, vol. 40, no. 7, pp. 23042315.
Allison, JD, Brown DS & Novo-Gradac, KJ 1991, ‘MINTEQA2/PRODEFA2, a geochemical assessment model for environmental systems: Version 3.0 User’s Manual’, EPA/600/3-91/021, United States Environmental Protection Agency, Washington, DC.
ANZECC & NHMRC 1992, Australian and New Zealand guidelines for the assessment and management of contaminated sites, Australian and New Zealand Environment and Conservation Council & National Health and Medical Research Council, Australia.
ANZECC & ARMCANZ 2000, National water quality management strategy. Australian and New Zealand guidelines for fresh and marine water quality, Australian and New Zealand Conservation Council and Agriculture & Agriculture and Resource Management Council of Australia and New Zealand.
Arnot, JA & Gobas, FAPC 2003, ‘A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs’, QSAR Combinatorial Science, vol. 22, pp. 337–345.
AS 2800 — 1985, Determination of particulate lead – high volume sampler gravimetric collection – flame atomic absorption spectrometric method, Standards Australia.
AS 2985 — 2004, Workplace atmospheres — method for sampling and gravimetric determination of respirable dust, Standards Australia.
AS 2986 — 1987, Workplace atmospheres — organic vapours: sampling by solid adsorption techniques, Standards Australia.
AS 3640 — 2009, Workplace atmospheres— method for sampling and gravimetric determination of inhalable dust, Standards Australia.
AS 3580 series Methods for sampling and analysis of ambient air, Standards Australia.
ASTM E1739–95 (1995a, re-approved 2002), Standard guide for risk based corrective action applied at petroleum release sites, American Society for Testing and Materials, Philadelphia, PA.
ASTM E1689–95 (1995b, re-approved 2008), Standard guide for developing conceptual site models for contaminated sites, American Society for Testing and Materials, Philadelphia, PA.
ASTM E2081–00 (2000), Standard guide for risk based corrective action, American Society for Testing and Materials, Philadelphia, PA.
ASTM E2531–06 (2006), Standard guide for development of conceptual site models and remediation strategies for light non-aqueous-phase liquids released to the subsurface, American Society for Testing and Materials, Philadelphia, PA.
ASTM E179203 (2011), Standard specification for wipe sampling materials for lead in surface dust, American Society for Testing and Materials, Philadelphia, PA.
ASTM E173995 (2010)e1, Standard Guide for Risk-Based Corrective Action Applied at Petroleum Release Sites, American Society for Testing and Materials, Philadelphia, PA.
ATSDR 2007, Toxicological profile for lead, Agency for Toxic Substances and Disease Registry, US Department of Health and Human Services, Atlanta, Georgia.
Butterworth, BE 1990, ‘Consideration of both genotoxic and non-genotoxic mechanisms in predicting carcinogenic potential‘, Mutation Research, vol. 239, pp. 117132.
Butterworth, BE 2007, ‘A mechanism-based cancer risk assessment for 1,4-dichlorobenzene’, Regulatory Toxicology and Pharmacology, vol. 49, pp. 138148.
Clewell, H 2005, ‘Use of mode of action in risk assessment: past, present, and future’, Regulatory Toxicology and Pharmacology, vol. 42, pp. 3014.
Country Health SA 2007, Port Pirie Environmental Health Service: strategic plan 20072010: safe healthy vibrant … every child matters, Country Health SA, Port Pirie, South Australia.
Cross, SJ & Taylor, ER 1996, Human exposure to soil contaminants through the consumption of home-grown produce, Contaminated sites monograph series, no. 6, South Australian Health Commission, Adelaide, Australia.
Crump, K 1995, ‘Calculation of the benchmark doses from continuous data’, Risk Analysis, vol. 15, pp. 7989.
Davis, GB, Trefry, MG & Patterson, BM 2004, Petroleum and solvent vapours: quantifying their behaviour, assessment and exposure, A report to the Western Australian Environment Department, CSIRO Land and Water, Australia.
Davis, GB, Trefry, MG & Patterson, BM 2009a, Petroleum vapour model comparison, CRC CARE Technical Report no. 9, CRC for Contamination Assessment and Remediation of the Environment, Adelaide, Australia.
Davis, GB, Patterson, BM & Trefry, MG 2009b, Biodegradation of petroleum hydrocarbon vapours, CRC CARE Technical Report no. 12, CRC for Contamination Assessment and Remediation of the Environment, Adelaide, Australia.
DEFRA & EA 2002, Contaminated land exposure assessment model (CLEA): technical basis and algorithms, Contaminated land report CLR 10, Department for Environment, Food and Rural Affairs & Environment Agency, Wallingford, UK.
EA 2005, The UK approach for evaluating human health risks from petroleum hydrocarbons in soils, Science report P5-080/TR3, Environment Agency, Bristol, UK.
EA 2006, Evaluation of models for predicting plant uptake of chemicals from soil, SC050021/SR, Environment Agency, Bristol, UK.
EA 2009, Updated technical background to the CLEA model, SC050021/SR3, Environment Agency, Bristol, UK.
El Saadi, O & Langley, A (eds) 1991, The health risk assessment and management of contaminated sites. Proceedings of a national workshop on the health risk assessment and management of contaminated sites, Contaminated sites monograph series, no. 1, South Australian Health Commission, Adelaide, South Australia.
enHealth 2004, Environmental Health Risk Assessment. Guidelines for assessing human health risks from environmental hazards, Department of Health and Ageing & enHealth Council, Canberra, Australia.
enHealth 2012a, Environmental health risk assessment. Guidelines for assessing human health risks from environmental hazards, enHealth Subcommittee (enHealth) of the Australian Health Protection Principal Committee, Canberra, Australia.
enHealth 2012b, Australian exposure factors guidance, Environmental Health Subcommittee (enHealth) of the Australian Health Protection Principal Committee, Canberra, Australia.
EPHC 2004, Time activity study: summary of findings, Environment Protection and Heritage Council , Australia.
Filipsson, AF, Sand, S, Nilsson, J & Victorin, K 2003, ‘The benchmark dose method — review of available models, and recommendation for application in health risk assessment’, Critical Reviews in Toxicology, vol. 33, no. 5, pp. 505542.
Friebel, E & Nadebaum, P 2011, Health screening levels for petroleum hydrocarbons in soil and groundwater. Part 1: Technical development document, CRC CARE Technical Report no. 10, CRC for Contamination Assessment and Remediation of the Environment, Adelaide, Australia.
Hamon, RE, McLaughlin, MJ, Gilkes, RJ, Rate, AW, Zarcinas, B, Robertson, A, Cozens, G, Radford, N & Bettenay, L 2004, ‘Geochemical indices allow estimation of heavy metal background concentrations in soils’, Global Biochemical Cycles, 10, GB1014.
Hamm, AK, Carter, WH, and Gennings, C 2005, ‘Analysis of an interaction threshold in a mixture of drugs and/or chemicals’, Statistics in Medicine, vol. 24, pp. 2493–2507.
Health Canada 1999, Revised Health Canada risk management framework. Draft for discussion, Health Canada, April.
Health Canada 2004, Federal contaminated Site Risk Assessment in Canada. Part I: Guidance on Human Health Preliminary Quantitative Risk Assessment (PQRA), Contaminated Sites Division, Health Canada.
IAP2 2000, Core values for the practice of public participation, International Association of Public Participation, CO, USA.
IARC 2006, ‘Preamble’, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, International Agency for Research on Cancer, World Health Organization, Lyon, France.
Johnson, PC & Ettinger, RA 1991, ‘Heuristic model for predicting the intrusion rate of contaminant vapours into buildings’, Environmental Science and Technology, vol. 25, pp. 14451452.
Jury, WA, Spencer, WF & Farmer, WJ 1983, ‘Behaviour assessment model for trace organics in soil: I. Model description’, Journal of Environmental Quality, vol. 12, pp. 558564.
Kelley, ME, Brauning, SE, Schoof, RA & Ruby, MA 2002, Assessing oral bioavailability of metals in soil, Battelle Press, Ohio, USA.
Kelly, KE 1991, The myth of 10-6 as a definition of ‘acceptable risk’, presented at the 84th Annual meeting and exhibition of the Air and Waste Management Association, Vancouver, BC, June 1621.
Lambert, JC & Lipscomb, JC 2007, ‘Mode of action as a determining factor in additivity models for chemical mixture risk assessment’, Regulatory Toxicology and Pharmacology, vol. 49, pp. 183194.
Langley, A & van Alphen, M (eds) 1993, ‘The health risk assessment and management of contaminated sites’, Proceedings of the second national workshop on the health risk assessment and management of contaminated sites, Contaminated sites monograph series no. 2, Canberra, Australia.
Langley, A, Markey, B & Hill, H (eds) 1996a, ‘The health risk assessment and management of contaminated sites’, Proceedings of the third national workshop on the health risk assessment and management of contaminated sites, Contaminated sites monograph series no. 5, South Australian Health Commission, Adelaide, Australia.
Langley, A, Imray, P, Lock, W & Hill, H (eds) 1996b, ‘The health risk assessment and management of contaminated sites’, Proceedings of the fourth national workshop on the health risk assessment and management of contaminated sites, Contaminated sites monograph series, South Australian Health Commission, Adelaide, Australia.
Langley, A, Gilbey, M & Kennedy, B (eds) 2003, ‘Health and environmental assessment of site contamination’, Proceedings of the fifth national workshop on the assessment of site contamination, National Environment Protection Council Service Corporation, Australia.
NEPC 1999, National Environment Protection (Assessment of Site Contamination) Measure, National Environment Protection Council, Australia.
NEPC 2000, National Environment Protection (Ambient Air Quality) Measure: The report of the risk assessment task force, National Environment Protection Council, Australia.
NEPC 2003, National Environment Protection (Ambient Air Quality) Measure, National Environment Protection Council, Australia.
NEPC 2004, National Environmental Protection (Air Toxics) Measure, National Environment Protection Council, Australia.
NEPC 2011, Methodology for Setting Air Quality Standards in Australia, National Environment Protection Council, Australia.
Ng, JC, Juhasz, AL, Smith, E & Naidu, R 2009, Contaminant bioavailability and bioaccessibility. Part 1: A scientific and technical review, CRC CARE Technical Report no. 14, CRC for Contamination Assessment and Remediation of the Environment, Adelaide, Australia.
NHMRC 2008, National water quality management strategy. Guidelines for managing risk in recreational water, National Health and Medical Research Council, Australia.
NHMRC, 2011, National water quality management strategy. Australian drinking water guidelines, National Health and Medical Research Council, Australia.
NJDEP 2005, Vapor intrusion guidance, New Jersey Department of Environmental Protection.
NRC 1994, Science and Judgement in Risk Assessment, Committee on Risk Assessment of Hazardous Air Pollutants, Board on Environmental Studies and Toxicology, Commission on Life Sciences, National Research Council, Washington, DC.
NRC 2008, Science and decisions: advancing risk assessment, National Research Council, National Academies Press, Washington, DC.
NSW DEC 1995, Contaminated sites sampling design guidelines, NSW Department of Environment and Conservation, New South Wales, Australia.
NSW DEC 2005, Approved methods for the modelling and assessment of air pollutants in NSW, NSW Department of Environment and Conservation, New South Wales.
NSW DECCW 2010, Vapour Intrusion: Technical Practice Note, Department of Environment, Climate Change and Water, New South Wales, Australia.
OECD 1998, ‘Section 4: Health effects’, OECD Guidelines for testing of chemicals, vol. 2, 10, Organisation for Economic Cooperation and Development, Paris.
Olszowy, H, Torr, P, Imray, P, Smith, P, Hegarty, J & Hastie, G 1995, Trace element concentrations in soils from rural and urban areas of Australia, Contaminated Sites monograph, no. 4, South Australian Health Commission, Adelaide, Australia.
Parkhurst, DL & Appelo, CAJ 1999, User’s Guide to PHREEQC, version 2: A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations, Water-resources investigations report 99-4259, United States Geological Survey.
Paustenbach, DJ 2000, ‘The practice of exposure assessment: a state-of-the-art review’, Toxicology and Environmental Health, Part B, vol. 3, pp. 179–291.
Priestly B 2009, ‘Review of risk assessment of chemical mixtures’, unpublished, provided to NEPC.
RIVM 2001, Guidance on deriving environmental risk limits, RIVM report 601501012, National Institute of Public Health and the Environment (RIVM), Bilthoven, The Netherlands.
SA EPA 2008, Site contamination – Determination of background concentrations, South Australia Environmental Protection Authority, Adelaide, Australia.
TPHCWG 1997a, Selection and use of representative TPH fractions based on fate and transport considerations, by Gustafson, J, Griffith Tell, J & Orem, D, Total Petroleum Hydrocarbon Criteria Working Group Series, vol. 3, Total Petroleum Hydrocarbons Working Group.
TPHCWG 1997b, Development of Fraction Specific Reference doses (RfDs) and reference concentrations (RfCs) for total petroleum hydrocarbons (TPH), by Edwards, DA, Total Petroleum Hydrocarbon Criteria Working Group Series, vol. 4, Total Petroleum Hydrocarbons Working Group.
TPHCWG 1998, Composition of petroleum mixtures, by Potter L & Simmons K, Total Petroleum Hydrocarbon Criteria Working Group Series, vol. 2, Total Petroleum Hydrocarbons Working Group.
US EPA 1989, Risk assessment guidance for Superfund, vol. I, Human health evaluation manual (Part A) Interim final report, EPA/540/1-89/002, United States Environmental Protection Agency, Washington, DC.
US EPA 1991a, Risk assessment guidance for Superfund, vol. I, Human health evaluation manual (Part B) Development of risk based preliminary remediation goals, United States Environmental Protection Agency, Washington, DC.
US EPA 1991b, Risk assessment guidance for Superfund, vol. I, Human health evaluation manual (Part C) Risk evaluation of remedial alternatives, United States Environmental Protection Agency, Washington, DC.
US EPA 1992, ‘Guidelines for exposure assessment’, Risk assessment forum, Federal Register 57(104)2288822938, United States Environmental Protection Agency, Washington, DC.
US EPA 1994, Methods of Derivation of Inhalation Reference Concentrations and Application of Inhalation Dosimetry. Office of Research and Development, Research Triangle Park, NC. EPA/600/8-90/066F.
http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=71993.
US EPA 1995a, Residential sampling for lead: protocols for dust and soil sampling, EPA 747/R–95/001, United States Environmental Protection Agency, Washington, DC.
US EPA 1995b, Sampling house dust for lead: basic concepts and literature review, EPA 747/ R-95/007, United States Environmental Protection Agency, Washington, DC.
US EPA 1995c, Guidance for risk characterisation, EPA 747/ R-95, United States Environmental Protection Agency, Washington, DC.
US EPA 1996, Soil screening guidance: user’s guide, EPA 540/R-96/018, United States Environmental Protection Agency, Washington, DC.
US EPA 1998, Risk assessment guidance for Superfund, vol. I, Human health evaluation manual (Part D) Standardised planning, reporting and review of Superfund risk assessments, United States Environmental Protection Agency, Washington, DC.
US EPA 1999, Risk assessment guidance for Superfund, vol. I, Human health evaluation manual (Supplement to Part A) Community involvement in Superfund risk assessments, United States Environmental Protection Agency, Washington, DC.
US EPA 1999, Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air, Second Edition, January 1999, and associated on-going reviews and updates at http://www.epa.gov/ttn/amtic/airtox.html
US EPA 2000, User’s guide for the NAPL-Screen and NAPL-ADV models for subsurface vapour intrusion into buildings, United States Environmental Protection Agency, Washington, DC.
US EPA 2001, Risk assessment guidance for Superfund, vol. III, (Part A), Process for conducting probabilistic risk assessment, EPA 540/R-02/002, United States Environmental Protection Agency, Washington, DC.
US EPA 2002a, Supplemental guidance for developing soil screening level for Superfund sites, OSWER, 9355.424, United States Environmental Protection Agency, Washington, DC.
US EPA 2002b, Role of background in the CERCLA clean-up program, OSWER 9285.6-07P, United States Environmental Protection Agency, Washington, DC.
US EPA 2002c, Guidance for comparing background and chemical concentrations in soil for CERCLA sites, United States Environmental Protection Agency, Washington, DC.
US EPA 2004a, User’s guide for evaluating subsurface vapor intrusion into buildings, United States Environmental Protection Agency, Washington, DC.
US EPA 2004b, Risk assessment guidance for Superfund, vol. I, Human health evaluation manual, (Part E), Supplemental guidance for dermal risk assessment, United States Environmental Protection Agency, Washington, DC.
US EPA 2005a, Guidelines for carcinogen risk assessment, EPA/630/P-03/001B, United States Environmental Protection Agency, Washington, DC.
US EPA 2006, Data quality assessment: statistical methods for practitioners, EPA/QA/G-9S, United States Environmental Protection Agency, Washington, DC.
US EPA 2007a, ProUCL version 4.00.04 user guide, EPA/600/R-07/38, United States Environmental Protection Agency, Washington, DC.
US EPA 2007b, Guidance for evaluating the oral bioavailability of metals in soils for use in human health risk assessment, OSWER 9285.7-80, United States Environmental Protection Agency, Washington, DC.
US EPA 2007c, Risk communication in action: the tools of message mapping, EPA/625/R-06/012, United States Environmental Protection Agency, Washington, DC.
US EPA 2009, Risk assessment guidance for Superfund, vol. I, Human health evaluation manual, (Part F), supplemental guidance for inhalation risk assessment, EPA/540/R-70/002, United States Environmental Protection Agency, Washington, DC.
US EPA 2011, Exposure factors handbook: 2011 Edition, EPA/600/R-090/052F, September 2011, United States Environmental Protection Agency, Washington, DC.
US EPA 2012a, Conceptual Site Model Scenarios for the Vapor Intrusion Pathway, EPA 530/R-10/003, February 2012, United States Environmental Protection Agency, Washington, DC.
US EPA 2012b, EPA’s Vapor Intrusion Database: Evaluation and Characterisation of Attenuation Factors for Chlorinated Volatile Organic Compounds and Residential Buildings, EPA 530/R-10/002, March 16 2012, United States Environmental Protection Agency, Washington, DC.
US EPA 2012c, Petroleum Hydrocarbons and Chlorinated Hydrocarbons Differ in their Potential for Vapor Intrusion, US EPA Office of Underground Storage Tanks, Washington, DC.
WA DEC 2006, Community Consultation, Contaminated Sites Management Series, Department of Environment and Conservation, Western Australia.
WA DoH 2009, Guidelines for the Assessment, Remediation and Management of Asbestos-Contaminated Sites in Western Australia, WA Department of Health, May 2009.
WHO 1994, Assessing human health risks of chemicals: derivation of guidance values for health-based exposure limits, Environmental Health Criteria 170, International Programme on Chemical Safety, World Health Organization, Geneva.
WHO 2000, Air quality guidelines for Europe, 2nd edn, WHO Regional publications, European series no. 91, World Health Organization, Copenhagen.
WHO 2004, IPCS risk assessment terminology: Harmonisation project, Document no. 1, World Health Organization.
WHO 2005, Principles of characterising and applying human exposure models: Harmonisation project, Document no. 3, World Health Organization.
WHO 2006, Elemental speciation in human health risk assessment, Environmental Health Criteria 234, World Health Organization.
WHO 2008, Guidance document on characterising and communicating uncertainty in exposure assessment (Part 1), and Hallmarks of data quality in chemical exposure assessment (Part 2): Harmonisation project, Document no. 6, World Health Organization.
WHO 2010, WHO Guidelines for Indoor Air Quality, Selected Pollutants, WHO Regional Office for Europe.
WHO 2011, Guidelines for drinking-water quality, 4th edn, World Health Organization, Geneva.
Additional Resources:
CCME 1996, Guidance manual for developing site-specific soil quality remediation objectives for contaminated sites in Canada. Canadian soil quality guidelines for the protection of environmental and human health, National Contaminated Sites Remediation Program, Canadian Council of Ministers of the Environment, Canada.
EA & BGS 2002a, In-vitro methods for the measurement of the oral bioaccessibility of selected metals and metalloids in soils: a critical review, R&D Technical report P5-062/TR/01, Environment Agency and British Geological Survey, Bristol, UK.
EA & BGS 2002b, Measurement of the oral bioaccessibility of arsenic in UK soils, R&D Technical report P5-062/TR/02, Environment Agency and British Geological Survey, Bristol, UK.
enHealth 2001, Exposure scenarios and exposure settings, 3rd edition, enHealth Council and Queensland Department of Health, Canberra, Australia.
IRTC 2007, Investigative approaches for typical scenarios: A supplement to ‘Vapour intrusion pathway, a practical guide’, Interstate Technology and Regulatory Council, World Health Organization, Washington, DC.
Kuzmack, AM & McGaughy, RE 1975, Quantitative risk assessment for community exposure to vinyl chloride, EPA Office of Planning and Management and Office of Health and Ecological Effects.
NSW DEC 2007, Approved methods for the sampling and analysis of air pollutants in NSW, NSW Department of Environment and Conservation, New South Wales, Australia.
ORNL Risk assessment information system, Oak Ridge National Laboratory, see website at http://risk.lsd.ornl.gov/index.shtml.
US EPA, Integrated exposure uptake biokinetic model for lead in children (IEUBK), United States Environmental Protection Agency, available online at
http://www.epa.gov/superfund/lead/products.htm.
US EPA, Integrated risk information system (IRIS), United States Environmental Protection Agency, see http://cfpub.epa.gov/ncea/iris/index.cfm.
US EPA, Regional screening levels, chemical specific parameters table, see http://www.epa.gov/region09/superfund/prg/.
Health risk assessment reports should be clear and transparent in their development; stating the objective of the assessment, setting the scene (CSM summary), and clearly identifying the data sources and assumptions that the assessment has been based upon. A clear visual image of the site, contaminant source locations, potential exposed populations and exposure pathways present at the site should be conveyed, for example, site plans and schematic CSM diagrams. The risk assessment report should provide a systematic approach for characterising the nature and magnitude of the risks associated with environmental health hazards with justification for decisions made throughout the report provided at each stage.
The objectives of this section are to:
Regulatory bodies should seek further information from consultants where reports:
Quantitative health risk assessments should follow the guidance provided in Schedules B4 and B7 and should be divided into the five key steps listed below to ensure logical reporting of the assessment:
The level of detail required in a risk assessment report should be appropriate to the complexity of the site and individual scenario requiring assessment. In order to make this judgment, the risk assessor and reviewer should consider:
Interpretation of site data and selection of input data used in the risk modelling is paramount to the outcome of the risk assessment and requires professional judgement. In the selection of appropriate representative input data, the assessor should consider the CSM and make it clear how the input values and modelling strategy (for example, use of fate and transport models) relate to the CSM.
The risk assessment should also consider detection limits and their appropriateness in relation to the screening criteria and/or toxicity of a substance. The risk assessor should justify the reasoning behind the input values chosen for any risk assessment.
The report language should be objective and avoid the use of subjective terms such as ‘heavy/medium/light contamination’ which can lead to confusion. In many parts of the risk assessment, expert judgement is necessary. It should be made clear where this is the case, and all assumptions should be identified and explained.
Further information on what is required and what to include in the five-step process that comprises the fundamentals of the risk assessment are presented in the following sub-sections.
Issues identification should be part of the introductory section of the risk assessment. Information on the following should be provided:
The relationship between the risk assessment and the risk management process should be made clear.
The data collection section relies on a well-designed site assessment developed with an understanding of potential exposure pathways/routes associated with past and present land use in mind. It is assumed here that the site investigation itself is presented as a separate document (or report section) which need not be repeated in the risk assessment though the raw data relied on in the risk assessment should be included as an appendix.
The data collection section should include the following:
The data evaluation section should include:
This section should include a clear discussion on the exposure scenarios likely to occur at the site, and whether the site-specific situation fits into the exposure scenarios characterised in Schedule B7. If these scenarios are not applicable or representative of the exposure scenario under assessment, an explanation together with behavioural and lifestyle assumptions and site assumptions should be provided within the report.
Software and mathematical algorithms used to calculate the contaminant intake should be referenced. It is only necessary to present equations if the risk assessment uses a method that is not published in full (for example, if amendments to algorithms are made). An explanation of why the model or approach selected is appropriate should be given.
All input variables should be presented and justified. Use of default assumptions should be justified.
All reasonable efforts should be employed to validate exposure models (model uncertainty) with field data (for example, soil vapour data to inform outputs from the model developed by Johnson and Ettinger (US EPA 2004a)) where possible.
In the hazard identification, a brief summary of the potential adverse effects of the contaminants of concern should be given. The summary should concentrate on the potential effects that are relevant to the contaminant in the context of the site and the exposure scenarios. Lengthy reviews of toxicology are not generally required. Clear presentation and referencing for physical and chemical properties is also required.
Doseresponse assessment involves selection of appropriate toxicity reference values. If toxicity reference values used are adopted from the relevant Schedule B7 appendix, then no additional explanation is required and a reference is sufficient. If toxicity reference values are selected for substances not included in Schedule B7, then explanation and justification of a similar order to that presented in Schedule B7 should be given.
This section needs to present the quantitative estimates of risk calculated through modelling and should provide an evaluation of the overall quality of the assessment and the degree of confidence the risk assessor has in the estimates of risk and conclusions drawn.
Risk characterisation should include a summary of key issues and conclusions, as well as describing the likelihood of adverse health effects. The summary should include a description of the assumptions made when conducting the risk assessment, together with the limitations and uncertainties associated with the risk assessment. The detailed risk calculations or model outputs should be presented in a manner that enables the calculations to be verified.
The conclusions should be presented in language that can be understood by non-specialists. The significance of the quantitative risk estimates should be explained in the context of the objectives of the project and the risk management decisions that need to be made.
Uncertainty analysis should identify sources of uncertainty in the risk assessment and quantify them as far as possible. A tabular presentation such as that given in enHealth (2004), Table 16, is considered likely to be suitable for many circumstances. The uncertainty analysis should be specific to the assessment undertaken; a generic appraisal of the uncertainties inherent in all risk assessments is not sufficient. The uncertainty analysis should identify the impact that the uncertainty may have on the outcome (using the sensitivity analysis where possible) and identify those uncertainties that are not included in the sensitivity analysis.
A sensitivity analysis should present the key quantifiable uncertainties and provide plausible ranges for each. The effect on the model outcome should be stated for each uncertainty (or set of related uncertainties). Commentary on the significance of uncertainties and variability should be given. A tabular format may be appropriate; an example is provided below.
Table 9. Example format for presentation of sensitivity analysis results
Variable | Range | Risk outcome | Sensitivity level / Comment | ||
Min | Max | Min | Max | ||
|
|
|
|
|
|
Acceptable daily intake (ADI) is the estimated maximum amount of a chemical expressed on a per kg body mass basis, to which individuals in a population may be exposed daily over their lifetimes without appreciable health risk. |
Acceptable risk is a risk management term. The acceptability of risk depends on scientific data, social, economic and political factors, and the perceived benefits arising from exposure to a chemical substance. |
Acute exposure is contact between a chemical substance and a target occurring over a short time, generally 14 days or less, with a single or repeated dose. Other terms such as ‘short-term exposure’ and ‘single-dose’ are also used. |
Adverse effect is change in the morphology, physiology, growth, development, reproduction, or life span of an organism, system, or population that results in an impairment of functional capacity, an impairment of the capacity to compensate for additional stress, or an increase in susceptibility to other influences. |
Aliphatic is a hydrocarbon compound that does not contain a benzene ring. Aliphatic compounds may be straight, branched or cyclic chains of carbon atoms. They may include double or triple bonds. Carbon atoms in the chain are also generally bonded to hydrogen atoms but other elements, for example, chlorine, sulphur and nitrogen, can also be present. |
Aromatic is a hydrocarbon containing one or more benzene rings. |
Background concentrations means the naturally occurring, ambient concentrations of substances in the local area of a site. |
Bioaccessibility is the fraction of a contaminant in an exposure medium that is soluble in the relevant physiological milieu (usually the gastrointestinal tract) and available for absorption. Generically, it is the ability for a chemical to come into contact with the absorbing surfaces in an organism. It is related to solubility and dissolution, since absorption usually can only occur from a liquid or gaseous phase and not from a solid phase. |
Bioavailability is a generic term defined as the fraction of a contaminant that is absorbed into the body following dermal contact, ingestion or inhalation. It is expressed as the ratio (or percentage) of the absorbed dose (systemic dose) to the administered dose. |
Cancer is a disease of heritable, somatic mutations affecting cell growth and differentiation; that is, genetic alterations incurred in the first damaged cells are acquired in subsequent cells after cell division within the same individual. |
Cancer slope factor (CSF) is the plausible upper-bound estimate of the probability of a response per unit of intake of a chemical substance over a lifetime. |
Carcinogen is a cancer-causing chemical substance/agent. |
Chemical of potential concern (COPC) is a chemical substance that is potentially site-related and whose data is of sufficient quality to be judged as potentially causing an adverse health effect. |
Chemical substance means any organic or inorganic substance, whether liquid, soil or gaseous. |
Chronic exposure is a continuous or repeated exposure contact between a chemical substance and a target. |
Clean-up level is a concentration of contaminant in soil or water derived for the purpose of providing an acceptable standard for remediation. May be risk-based or modified by considerations of feasibility, practicality, acceptability, timescale and cost. |
Concentration is the amount of material or chemical substance dissolved or contained in unit quantity in a given medium or system. |
Conceptual site model (CSM) is a description of a site including the environmental setting, geological, hydrogeological and soil characteristics together with nature and distribution of contaminants. Potentially exposed populations and exposure pathways are identified. Presentation is usually graphical or tabular with accompanying explanatory text. |
Contact volume is a volume containing the mass of chemical substance that contacts the exposure surface. |
Contaminant is any chemical existing in the environment above background levels and representing, or potentially representing, an adverse health or environmental risk. |
Contamination means the condition of land or water where any chemical substance or waste has been added as a direct or indirect result of human activity at above background level and represents, or potentially represents, an adverse health or environmental impact. |
Critical effect is the adverse effect(s) judged to be the most appropriate for determining the tolerable intake. |
Data quality objectives (DQOs) involve the establishment of the amount, nature and quality of data required to complete a specific risk assessment. |
Dose is the total amount of a chemical administered to, taken up by, or absorbed by an organism, system, or population. |
Doseresponse curve is the graphical representation of a doseresponse relationship. |
Doseresponse is the relationship between the amount of chemical administered to, taken up by, or absorbed by an organism, system, or population and the change developed in that organism, system, or population in reaction to the chemical substance. |
Effect is change in the state or dynamics of an organism, system, or population caused by exposure to a chemical. |
Expert/professional judgement is the opinion of an authoritative person on a particular subject. |
Exposed population comprises the people who may be exposed to the contaminant. Synonymous with ‘receptor’. |
Exposure assessment is the evaluation of the exposure of an organism, system, or population to a chemical (and its derivatives). |
Exposure is the concentration or amount of a particular chemical that reaches a target organism, or system, or population in a specific frequency for a defined duration. |
Exposure concentration is the exposure mass divided by the contact volume or the exposure mass divided by the mass of contact volume, depending on the medium. |
Exposure duration is the length of time over which continuous or intermittent contacts occur between a chemical and the exposed population. |
Exposure event is the occurrence of continuous contact between chemical and exposed population. |
Exposure frequency is the number of exposure events within an exposure duration. |
Exposure model is a conceptual or mathematical representation of the exposure process. |
Exposure pathway is the means by which a contaminant makes contact with the exposed population. |
Exposure route is the way in which a chemical substance enters a target after contact (for example, ingestion, inhalation or dermal absorption). |
Exposure scenario is a set of conditions or assumptions about sources, exposure pathways, concentration of contaminants involved, and exposed population (i.e. numbers, characteristics, habits) used in the evaluation and quantification of exposure(s) in a given situation. |
Genotoxic chemicals are those for which there is adequate evidence of the potential to interact with, and/or modify the function of genetic material and which has the ability to induce tumours via a mechanism involving direct damage to DNA. |
Hazard identification is the identification of the type and nature of adverse effects that a contaminant has an inherent capacity to cause to an exposed population. |
Hazard indices/index (HI) is the sum(s) of at least two hazard quotients. It is noted that the WHO is moving towards the use of risk indices/index (RI). |
Hazard is the inherent property of a contaminant or situation having the potential to cause adverse effects when a population may be exposed to that contaminant. |
Hazard quotient (HQ) is the ratio of the mean daily intake to the reference dose or tolerable daily intake for threshold exposure. It is noted that the WHO is moving towards the use of risk quotient (RQ). |
Health risk assessment (HRA) is the process of estimating the potential impact of a chemical, biological or physical agent on a specified human population system under a specific set of conditions. |
Health risk management is the process of evaluating and implementing appropriate options to address risks identified from health risk assessments. The decision-making will incorporate scientific, social, economic and political information. |
Health investigation levels (HILs) mean the concentration of a contaminant above which further appropriate investigation and evaluation will be required to ensure the protection of human health. |
Intake is the total amount of contaminant (or dose) taken into the body by the exposure route. |
Non-aqueous phase liquid (NAPL) is a chemical substance that is insoluble or only slightly soluble in water that exists as a separate liquid phase in environmental media. The free liquid phase of a chemical substance, i.e. not dissolved in water or adsorbed to soil. |
Non-genotoxic carcinogen is a chemical substance that induces tumours via a mechanism that does not involve direct damage to genetic material (DNA). |
Pica is a behaviour exhibited occasionally by young children and rarely by adults, characterised by the deliberate ingestion of non-nutritive substances, such as soil. Habitual or repetitive pica specifically involving soil-eating behaviour is uncommon. |
Reference dose is an estimate of the daily exposure dose that is likely to be without deleterious effect even if continued exposure occurs over a lifetime. Equivalent in meaning to tolerable daily intake and acceptable daily intake. |
Remediation is the cleaning up of contaminated land. |
Response is the change developed in the state of dynamics of an organism, system, or population in reaction to exposure to a chemical substance. |
Risk means the probability in a certain timeframe that an adverse outcome will occur in a person, a group of people, plants, animals and/or the ecology of a specified area that is exposed to a particular dose or concentration of a chemical substance, i.e. it depends on both the level of toxicity of the chemical substance and the level of exposure to it. |
Risk assessment is the process of estimating the potential impact of a chemical, physical, microbiological or psychosocial hazard on a specified human population or ecological system under a specific set of conditions and for a certain timeframe. |
Risk characterisation is the qualitative and, wherever possible, quantitative determination, including attendant uncertainties, of the probability of occurrence of known and potential adverse effects of a contaminant in a given organism, system, or population, under defined exposure conditions. |
Risk communication is the interactive exchange of information about health and environmental risks amongst risk assessors, managers, news media, interested groups, and the general public. |
Risk estimation is the quantification of the probability, including attendant uncertainties, that specific adverse effects will occur in an organism, system, or population due to actual or predicted exposure. |
Risk evaluation is the establishment of a qualitative or quantitative relationship between risks and benefits of exposure to a chemical substance, involving the complex process of determining significance of the identified hazards and estimated risks to the system concerned or affected by exposure. Risk evaluation is an element of risk management. Risk evaluation is synonymous with risk-benefit evaluation. |
Risk management is a decision-making process involving consideration of political, social, economic, and technical factors with relevant risk assessment information relating to a hazard to determine an appropriate course of action. |
Safety involves the practical certainty that adverse effects will not result from exposure to a chemical substance under defined circumstances. It is the reciprocal of risk. |
Screening criteria are concentration values used in screening. Usually published for the purpose by an authoritative body (for example, HILs) or derived according to a specified methodology. |
Screening is the process of comparison of site data to screening criteria to obtain a rapid assessment of contaminants of potential concern. |
Sensitive groups are sub-populations with both susceptibility and vulnerability factors. |
Sensitivity analysis is the process of changing one variable while leaving the others constant and determining the effect on the output. The procedure involves fixing each uncertain quantity, one at a time, at its credible lower bound and then its upper bound (holding all other at their medians), and then computing the outcomes for each combination of values. It can be used to test the effects of both uncertainty and variability in input values. |
Site means the parcel of land being assessed for contamination. |
Site-specific target levels are risk-based concentration values derived using Tier 2 or Tier 3 exposure modelling. May be used as criteria for further assessment or as clean-up levels. |
Source is the contaminant that is considered to represent a potential risk requiring assessment. |
Sub-chronic exposure is a contact between a chemical substance and a target of intermediate duration between acute and chronic. Different bodies vary on their definitions of the duration of ‘sub-chronic’ exposure, since it varies with species. US EPA uses up to 10% of an organism’s lifetime (enHealth 2012a); however, between 3 and 6 months is often used when discussing sub-chronic exposure to people. |
Susceptibility refers to intrinsic biological factors that can increase the health risk of an individual at a given exposure level. Examples of susceptibility factors include genetic factors, late-age and early-life, prior or existing disease. |
Threshold is the dose or exposure concentration of a chemical substance below which a stated effect is not observed or expected to occur. |
Tier 1 assessment is a risk-based analysis comparing site data with generic published screening criteria for various property uses (for example, residential, commercial and industrial). This tier has the lowest data requirement, generic exposure assumptions, and applies the most conservative criteria. |
Tier 2 assessment is a site-specific assessment in which risks to potentially exposed populations are assessed using site-specific data on pathways, land uses and the characteristics of the exposed populations. A Tier 2 evaluation usually involves the use of a quantitative exposure model. A Tier 2 evaluation is more complex than a Tier 1 evaluation and requires more site-specific information. As a result, a health protective effect will be achieved with a lower level of conservatism. |
Tier 3 assessment is a further step from a Tier 2 assessment and looks in more detail at specific risk-driving factors. This often involves additional data collection, and may incorporate more sophisticated modelling techniques. |
Tolerable daily intake (TDI) is analogous to acceptable daily intake. The term ‘tolerable’ is used for substances that are not deliberately added, such as contaminants in food and water. |
Toxicity is the inherent property of a chemical substance to cause an adverse biological effect. |
Toxicity reference value (TRV) is a measure of tolerable intake or acceptable risk. The TRV may be associated with either a threshold (i.e. ADI, TDI, TC or reference dose) or non-threshold (i.e. slope factor or unit risk) dose-response relationship. |
Uncertainty is a lack of, or incomplete, information or knowledge. |
Unit risk is an expression of the incremental risk associated with increase in exposure by a single unit of exposure measure. It may also be expressed as the plausible upper bound estimate of the probability of a response from a chemical over a lifetime. It is derived from the slope of the linearised doseresponse relationship and usually expressed in units of concentration for a specified medium (e.g. incremental risk per μg/m3 in air). |
Uptake is the amount of contaminant that enters the body through a barrier such as the skin, lungs or gut lining. Uptake is generally less than intake because not all the contaminant that enters the lungs or gut, or contacts the skin, is absorbed. |
Vadose zone is the portion of the sub-surface between the water table and the ground surface, also termed the unsaturated zone. Soil pore space in the vadose zone is only partially occupied by water, which is held in place by capillary forces and adhesion to soil particles. |
Variability describes true differences in attributes or values due to diversity or heterogeneity. |
Vulnerability refers to human populations at higher risk due to environmental factors; examples of vulnerability factors include poverty, malnutrition, poor sanitation, climate change, and stress associated with mental health diseases. |
ABS fraction of contaminant absorbed
ADI acceptable daily intake
ADWG Australian Drinking Water Guidelines
AF adherence factor of soil to skin
AM arithmetic mean
ANZECC Australian and New Zealand Environment and Conservation Council
AS Australian Standard
ASTM American Society for Testing and Materials
AT averaging time
ATSDR Agency for Toxic Substances and Disease Registry
BMD benchmark dose
BW body weight
CA concentration in air
CDI chronic daily intake
CF concentration factor
CRC CARE Cooperative Research Centre for Contamination Assessment and Remediation of the Environment
Cs concentration in soil
Cw concentration in water
Csat saturation limit concentration
CSF cancer slope factor
DAF dermal absorption factor
DEFRA Department of Environment, Food and Rural Affairs (UK)
DQOs data quality objectives
EA Environment Agency (England and Wales)
EC exposure concentration
ED exposure duration
EF exposure frequency
FI fraction ingested from contaminated source
GAF gastrointestinal absorption factor
GIL groundwater investigation level
HAP hazardous air pollutants
HEAST health effects assessment summary tables (US EPA)
HI hazard index
HIL health investigation level
HQ hazard quotient
HSL health screening level
IAP2 International Association of Public Participation
IARC International Agency for Research on Cancer
IEUBK integrated exposure uptake biokinetic model (US EPA)
ILCR increased lifetime cancer risk
IQ intelligence quotient
IR ingestion rate or inhalation rate (as referenced in equations)
IRIS Integrated Risk Information System (US EPA)
ITRC Interstate Technology & Regulatory Council
JECFA Joint FAO/WHO Expert Committee on Food Additives
LOAEL lowest observable adverse effect level
LOD limit of detection
LOR limit of reporting
MRLs minimal risk levels
NAPL non-aqueous phase liquid
NEPC National Environment Protection Council
NHMRC National Health and Medical Research Council
NICNAS National Industrial Chemicals Notification and Assessment Scheme
NJ DEP New Jersey Department of Environmental Protection
NOAEL no observable adverse effect level
NRC National Research Council
NSW DEC New South Wales Department of Environment and Conservation (now NSW Environment Protection Authority)
OECD Organisation for Economic Cooperation and Development
PAHs polycyclic aromatic hydrocarbons
PBTs persistent bioaccumulative and toxic pollutants
PCBs polychlorinated biphenyls
PCE perchloroethene
PEF particulate emission factor
PPRTV provisional peer reviewed toxicity value (US EPA)
RAGS Risk Assessment Guidance for Superfund (US EPA)
RBALP Relative Bioavailability Leaching Procedure
REL Reference exposure level
RfC reference concentration
RfD reference dose
RI risk index
RQ risk quotient
SA skin area
SA EPA South Australia Environment Protection Agency
SBRC Solubility Bioavailability Research Consortium
SD standard deviation
TC tolerable concentration in air
TBT tributyltin
TCA trichlorethane
TCE trichloroethene
TDI tolerable daily intake
TEF toxicity equivalence factor
TPH total petroleum hydrocarbons
TPHCWG Total Petroleum Hydrocarbon Criteria Working Group
TRV toxicity reference value
UCL upper confidence limit
URF unit risk factor
US EPA United States Environmental Protection Agency
US NOAA United States National Oceanic and Atmospheric Administration
WHO World Health Organization