
![]()

![]()


![]()

![]()

Page
1 Introduction
2 EIL derivation methodology
2.1 Overview of the EIL derivation methodology
2.2 Levels of protection
2.2.1 Levels of protection for specific land uses
2.2.1.1 National parks and areas with high ecological value
2.2.1.2 Urban residential and public open space
2.2.1.3 Commercial and industrial land
2.2.1.4 Agricultural land
2.3 Determining the most important exposure pathways
2.3.1 Exposure pathway assessment for organic contaminants
2.3.1.1 Half-life
2.3.1.2 Henry’s law constant
2.3.1.3 Octanol-water partition
2.3.1.4 Overview of the main exposure pathways for organic contaminants
2.3.2 Exposure pathway assessment for inorganic contaminants
2.3.2.1 Biomagnification
2.3.2.2 Henry’s law constant
2.3.2.3 Overview of main exposure pathways for inorganic contaminants
2.4 Derivation of EIL values
2.4.1 Collation and screening of data
2.4.1.1 Toxicity data collation
2.4.1.2 Quantitative structureactivity relationships
2.4.1.3 Quantitative activityactivity relationships
2.4.1.4 Equilibrium partitioning method
2.4.1.5 Screening and selection of toxicity data
2.4.2 Standardisation of the toxicity data
2.4.2.1 Measures of toxicity
2.4.2.2 Conversion from total to added concentrations
2.4.2.3 Duration of exposure
2.4.2.4 The use of toxicity data for endemic or overseas species
2.4.3 Incorporation of an ageing and leaching factor
2.4.4 Comparison of available toxicity data to the minimum data requirements
2.4.5 Calculation of the added contaminant limit using a species sensitivity distribution approach
2.4.6 Normalisation of toxicity data to an Australian reference soil
2.4.7 Calculation of the added contaminant level using an assessment factor approach
2.4.8 Accounting for secondary poisoning and biomagnification
2.4.9 Calculation of the ambient background concentrations
2.4.9.1 Inorganic contaminants
2.4.9.2 Organic contaminants
2.4.10 Calculation of the EIL
2.4.11 The reliability of the EIL
2.4.12 Evaluation of the appropriateness of the derived EILs
2.4.13 Strengths and limitations of EIL derivation methodology
2.4.13.1 Strengths
2.4.13.2 Limitations
3 Technical notes on methods used in the EIL derivation methodology
3.1 Methods to account for the effect of soil characteristics on toxicity and bioavailability
3.1.1 Chemical estimates of bioavailability
3.1.2 Normalisation relationships
3.1.3 Normalisation of toxicity data to a reference soil
3.2 Methods to calculate soil quality guidelines
3.2.1 Species sensitivity distribution methods
3.2.2 How do SSD methods work?
3.2.2.1 Criticisms
3.2.2.2 Strengths and limitations
3.2.3 Assessment factor methods
3.2.3.1 Criticisms
3.2.3.2 Strengths and weaknesses
3.2.4 Geometric mean methodology of the US EPA
3.2.4.1 Strengths and limitations
3.2.5 Methods for calculating EILs
3.2.6 Secondary poisoning and biomagnification
3.2.7 Methods for accounting for secondary poisoning
3.2.8 Using biomagnification algorithms
3.2.9 Using a default biomagnification factor
3.2.10 Increasing the percentage of species to be protected
3.3 Determining ambient background concentrations
3.3.1 Inorganics
3.3.2 Background concentration models
3.3.3 Organics
4 Bibliography
5 Appendices
5.1 Appendix A: Review and comparison of frameworks for deriving soil quality guidelines in other countries
5.1.1 A1: USA
5.1.2 A2: The Netherlands
5.1.3 A3: Canada
5.1.4 A4: EU and UK
5.1.5 A5: Germany
5.1.6 A6: New Zealand
5.2 Appendix B: method for deriving EILs that protect aquatic ecosystems
5.2.1 Determining the leaching potential of inorganic contaminants
5.2.2 Determining the leaching potential of organic contaminants
5.2.3 Calculation of EILs that protect aquatic ecosystems
5.2.3.1 Inorganic contaminants
5.2.3.2 Organic contaminants
5.3 Appendix C: Methods for determining the bioavailability of contaminants and how this could be incorporated into the ERA framework
6 Glossary
7 Shortened forms
This guideline presents the methodology for deriving terrestrial ecological investigation levels (EILs) for three groups of land uses: (1) areas of ecological significance (2) urban residential/public open space, and (3) commercial/industrial. The methodology was developed to protect soil processes, soil biota (flora and fauna) and terrestrial invertebrates and vertebrates and is presented in this Schedule. Also addressed is the strength and limitations of the EIL derivation methodology. Technical notes on the methods used in the methodology are also provided. In developing the EIL derivation methodology, the approaches used by other countries were investigated and a summary of these is presented in Appendix A.
This methodology should be considered together with National water quality management strategy – Australian and New Zealand guidelines for fresh and marine water (ANZECC& ARMCANZ 2000) where there are risks of impact to the aquatic ecosystem.
The methodology was developed being cognisant of both the methods used in other jurisdictions and of the existing methods used in Australia to derive water and sediment quality guidelines (ANZECC & ARMCANZ 2000; Simpson et al. 2005; Simpson & Batley 2007). The methodology is flexible and can deal with a variety of different land uses, risk pathways and toxicity data. It could be used to derive not just EILs but also other soil quality guidelines (SQGs) that have different purposes and/or different land uses. Examples of other SQGs include negligible risk target values, clean-up guidelines (goals that a site remediation must meet), intervention values (guidelines that, if exceeded, require immediate action in the form of remediation), and agricultural guidelines (guidelines to protect the long-term sustainability of agricultural land). The same basic methodology could also be used to derive guidelines for contaminants in products that are added to soil such as soil amendments, biosolids, fertilisers and re-use of wastes or by-products. In fact, guidelines for cadmium, copper and zinc for Australian biosolids applied to agricultural land have been developed using a very similar method (Warne et al. 2007, Heemsbergen et al. 2009). While the methodology can be used to derive other SQGs, this guideline will henceforth only focus on EILs.
An overview of the EIL derivation methodology is given in Figure 1. It consists of three main steps:
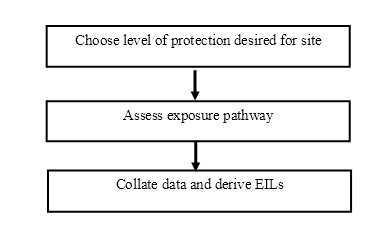

Figure 1. Overview of the methodology for the derivation of EILs.
Selecting the level of protection to be provided to a site or soil is one of the most important steps in the EIL derivation methodology.
The level of protection provided will depend on:
The land use-based approach has been adopted by several countries (for example, Germany and Canada). The Canadian soil quality guidelines (CCME 2006, Appendix A3) include four land-use types—agricultural, residential/parkland, commercial and industrial. Each land use has a list of relevant ecological receptors of concern to be included in the derivation of the Canadian SQGs. Furthermore, at industrial and commercial sites, a low level of adverse effects would be expected to occur in less than half of the species in the terrestrial community, as the CCME set the species protection level at 50%. Therefore, each land use type has its own SQG (CCME 2006).
The Australian and New Zealand water quality guidelines (WQGs) (ANZECC and ARMCANZ 2000) include a similar approach, which provides different levels of protection (that is, percentage of species) to aquatic ecosystems depending on how pristine the ecosystem is (that is, their current conservation status).
For pristine and thereby high conservation value ecosystems, slightly to moderately disturbed, and highly disturbed ecosystems, the default levels of protection in Australian aquatic ecosystems are 99% (PC99), 95% (PC95) and 90% (PC90) or 80% (PC80) of species, respectively (ANZECC & ARMCANZ 2000).
The EIL derivation methodology was used to derive a series of SQGs for eight contaminants using three different sets of toxicity data and thus providing three different levels of protection (Schedule B5c). For practicable application, the National Environment Protection (Assessment of Site Contamination) Measure (the NEPM) has adopted a combination of lowest observed effect concentration (LOEC) and 30% effect concentration data (EC30) for derivation of the EILs[1]. For further information about this toxicity data refer to the Glossary and relevant Section.
For all land uses (urban residential, public open space, commercial, industrial, agricultural, national parks/areas with high ecological value), with the exception of agriculture (see paragraph below on agricultural land), the following ecological receptors are relevant:
Henceforth, the above list of protected organisms will be referred to as ‘species and soil microbial processes’.
The level of protection provided varies depending on the land use and whether the contaminant in question biomagnifies. Different levels of protection are aimed at protecting certain percentages of species and soil microbial processes[2]. The percentages of species to be protected will apply to the land uses irrespective of the purpose of the SQG. If a protection level is set at 80%, then theoretically 20% of the species and soil processes are at risk of experiencing adverse effects.
The toxic effects that this 20% of species/soil processes may experience will vary depending on the type of toxicity data that was used to derive the SQG. For example, for SQGs derived using NOEC (no observed effect concentration) or EC10 data, the potentially affected 20% of species/soil processes would experience toxic effects that were not significantly different to the controls or up to a 10% effect respectively. For SQGs based on EC50 data, the potentially affected 20% of species/processes could experience a 50% effect.
Biomagnification and the corresponding levels of protection should be enacted only when:
A summary of the percentages of species and soil microbial processes to be protected in soil with different land uses is given in Table 1 below.
Table 1. Percentage of species and soil processes to be protected for different land uses
Land use | Standard % protection | Biomagnificationa % protection |
Urban residential | 80 | 85b |
Public open space | 80 | 85b |
Commercial | 60 | 65c |
Industrial | 60 | 65c |
Agricultural | 95d and 80e | 98c,d and 85c,e |
Areas of ecological significance | 99 | 99 |
a if a contaminant meets the criteria for biomagnification, b if surface area exceeds 250 m2, c if surface area exceeds 1,000 m2, d agricultural crops, e for soil processes and terrestrial fauna.
The level of protection for some of the land uses are the same. Therefore, some of the land uses have been combined. Thus, in essence, there are only four different land uses: 1) national park/area with high ecological value, 2) urban residential/public open space, 3) commercial/industrial, and 4) agricultural. The NEPM focuses on the first three groups.
National parks and areas with high ecological value are near-pristine ecosystems and should remain in that condition. As far as possible, it should be ensured that these ecosystems are not affected by soil contamination. Therefore, the appropriate level of protection is 99% of species. As this is the maximum percentage of protection possible (due to the statistical method used to calculate SQG), 99% is also the species protection setting for contaminants that biomagnify.
Henceforth, this grouping of land uses will be referred to as ‘urban residential’. Urban residential lands are not pristine, rather, they are extensively modified, but they still retain many important functions and species. Stakeholders would expect these to be maintained. For example, it would be reasonable to expect that such land uses should sustain plant growth of both introduced (ornamental) and native species. To ensure viable growth of plant species, not only should plant toxicity data be considered but also soil health (for example, nutrient cycling and microbial functions). Nutrient cycling in soil ecosystems is essential for plant growth and therefore both microorganisms and soil invertebrates should be protected. Microorganisms are responsible for many processes regarding nutrient cycling—decomposition of organic matter, and N and P cycling processes (Marschner & Rengel 2007). Soil invertebrates have a number of important functions, including interacting with microorganisms regarding nutrient cycling, and modifying soil structure. In addition, many birds and small terrestrial animals feed on plants and soil invertebrates in urban areas. Therefore, secondary poisoning for some contaminants should be assessed to ensure adequate protection is provided to organisms high in urban food chains.
As urban residential lands are modified ecosystems, it would not be warranted or realistic to protect 95% of species and functions. Yet a reasonably high degree of protection is required in order to maintain the desired receptors and ecological functions. It has therefore been decided to protect 80% of species and soil microbial processes appropriate to this land use. For contaminants with a potential for biomagnification, the percentage of species protected should be raised by 5% to 85%.
Henceforth, these two land uses will be referred to as commercial/industrial land use. Ecosystems in commercial/industrial lands can be highly artificial. However, soils should still support the basic soil processes and should be able to recover if land use changes. Therefore, 60% of species will be protected for non-biomagnifying contaminants present in commercial/industrial land and 65% for contaminants that show biomagnification potential.
The protection of crop species is vital to maintaining the sustainability of agricultural land and therefore 95% of the crop and grass species will be protected for this land use. Other plant species will not be used in the derivation of agricultural SQGs and therefore it will not be known what level of protection is provided by the SQG to native flora. Soil processes and soil invertebrates are highly important to ensure nutrient cycling to sustain crop species. However, tillage and the use of pesticides/herbicides make it unrealistic to protect 95% of soil processes and soil invertebrates and therefore only 80% of these will be protected. If a contaminant shows biomagnification potential, the percentage of species protected should be raised to 98% for crop species and 85% for soil processes and soil invertebrates. The lower of these two derived SQG values has been adopted as the agricultural SQG, and is included for information purposes only.
It is important to determine the relevant exposure pathways for the combination of specific contaminants at a specific land use. For the sake of simplicity, many of the exposure pathways have been grouped into three pathways:
The importance of the various exposure pathways can be determined by categorising the physicochemical properties of the toxicant and those of the receiving soil that control the environmental fate of chemicals. An overview of compartments within soil and the physicochemical properties that determine the fate of contaminants is given in Box 2 below. Several of the physicochemical properties shown are soil-dependent, for example, soil pH, cation exchange capacity, organic matter, clay content and dissolved organic carbon.
However, others are physicochemical properties of the contaminant itself, for example, partitioning between octanol and water (Kow), its soil to water partition coefficient (Kd), Henry’s law constant (H). These physicochemical properties can be used to determine the most important exposure pathways for contaminants. Organic and inorganic contaminants have different physicochemical properties that control their environmental fate and therefore different schemes for assessing exposure routes have been developed.
The EIL derivation methodology aims to protect soil and terrestrial species and soil processes. Potential off-site migration and its potential impacts are not included in the methodology. A recommended method for deriving EILs and/or other SQGs that also protects aquatic ecosystems is presented as an Appendix. Another issue that was considered for incorporation into the EIL derivation methodology was the bioavailability of the contaminants before addition to soil; for example, soluble contaminants versus those bound in insoluble forms. While this is a central issue in the management of contamination, it is not currently possible to incorporate this into the derivation of EILs and/or SQGs and the derivation assumes contaminants are 100% bioavailable. Some information on potential methods for assessing bioavailability and how it could be incorporated into a more detailed site-specific risk assessment is provided as an Appendix.
Box 1. Overview of potential exposure pathways in terrestrial ecosystems
|
Exposure pathways
The exposure pathways can be grouped together:
|
Box 2. Soil compartments, routes of environmental exposure and the key physicochemical properties that govern the distribution of a contaminant `
|
Properties controlling the environmental fate and exposure routes of chemicals:
|
The environmental fate of organic contaminants is largely controlled by three physicochemical properties:
The half-life (t½) of a contaminant is a measure of persistence of the contaminant in the environment. It represents the time taken for 50% of the contaminant to be lost from the environment. The loss may occur through biodegradation (microbially mediated degradation) or abiotic pathways (hydrolysis, oxidation, reduction, etc.). The more persistent a contaminant in the environment (that is, larger t½), the longer is the potential exposure time of species to the contaminant and the more deleterious the effects that could occur[3].
In order to classify contaminants in terms of their half-lives, the most relevant comparison is their persistence (based on half-life) to the generation time of soil organisms. Soil organisms do vary greatly, with some microbes having generation times of hours, while earthworms have a generation time of approximately one year. A generic generation time of three months for soil organisms (microorganisms were not considered) was selected and the resulting categories of biodegradation rates can be found in Table 2 below.
Half-lives of contaminants depend on the soil physicochemical properties and therefore preference should be given on half-life values based on Australian soils. However, if this information is not available for Australian soils, then appropriate overseas studies can be used.
Table 2. Biodegradation rates, half-lives and the classification to be used in assessing the importance of the various exposure pathways for organic contaminants.
94% of contaminant degraded in (months) | t1/2 (days) | t1/2 Classification |
<3 | <22.5 | Fast (F) |
36 | 22.545 | Moderately fast (M) |
>6 | >45 | Slow (S) |
Henry’s law constant (H) is a measure of the volatility of the contaminant. The higher the volatility (or value of H) the more of the contaminant will volatilise and be found in the soil air and in the atmosphere. H is a temperature-dependent constant.
Together with the t1/2 of the contaminant, H is used to assess the transfer and persistence of the contaminant in the soil, as vapour transport for many contaminants may constitute an important pathway of loss and exposure to organisms.
Several researchers have used different cut-off values of H to class contaminants into volatile and non-volatile categories but, in most cases, for aquatic environments. Jury et al. (1983, 1984) categorised the behaviour of trace organic contaminants in soils using H (among other properties) and this is useful to assess the importance of the various exposure pathways for organic contaminants (see Table 3 below). Jury et al. (1983) used the Henry’s law constant in dimensionless form as the ratio of concentration in the gas phase to concentration in the liquid phase, both in units of molar concentration, that is, H = (molar concentration in air)/(molar concentration in water)[4]. This is the most relevant form for estimation of the mass distribution of a chemical.
The dimensionless form of H based on concentrations (on a molar concentration basis) is the most commonly used of the dimensionless values (Staudinger & Roberts, 1996). The US EPA has published a calculator where Henry’s law constant, H, can be estimated in different unit forms and at different temperatures. This can be accessed at www.epa.gov/athens/learn2model/part-two/onsite/esthenry.htm.
Table 3. Henry’s law constant (H dimensionless) values to be used in assessing the importance of the various exposure pathways for organic contaminants
Henry’s constant value (cm3 air/cm3 solution) | Classification |
>2.5 x 10 -3 | Highly volatile (H) |
2.5 x 10-72.5 x 10 -5 | Moderately volatile (M) |
<2.5 x 10 -7 | Not volatile (L) |
The octanolwater partition (Kow) is the ratio of the concentration of a contaminant that is dissolved in n-octanol to that dissolved in water at equilibrium and at a specified temperature. It is used as a surrogate to estimate the potential for contaminants to accumulate in tissue, both plant and animal (Connell 1989, Posthumus & Slooff 2001). The Kow values can often be so large that the values are usually expressed as the logarithm to base 10 (that is, log Kow). Contaminants with high log Kow values are more likely to accumulate in plants and soil invertebrates than contaminants with low Kow values (Connell 1989, Posthumus & Slooff 2001). If further magnification of these contaminants occurs in the food chain, the predators might experience toxicity while its prey does not. This effect is known as secondary poisoning.
Contaminants with log Kow values below 4 are not considered to biomagnify, while highly fat soluble, lipophilic contaminants with log Kow values equal to or greater than 4 are most likely to biomagnify. For most contaminants, it is expected that metabolism, excretion and degradation rates exceed the bioaccumulation rates at concentrations equivalent to the trigger values for protecting aquatic ecosystems (ANZECC & ARMCANZ 2000). Hence, only for contaminants with log Kow values equal to or greater than 4 should secondary poisoning be considered. This approach is also consistent with the starting point to consider biomagnification used in the Australian and New Zealand WQGs (ANZECC & ARMCANZ 2000).
For the purpose of this methodology, the log Kow values of contaminants are divided into two classes. These are:
Table 4 below presents the various combinations of the three physicochemical properties of organic contaminants described above and the resulting two exposure routes that are considered the most important for deriving EILs and/or SQGs.
Slowly degrading contaminants (that is, t1/2 = slow, Table 2) with high log Kow values and low H will have biomagnification as the most important exposure pathway followed by direct toxicity. If, however, these slowly degrading, high log Kow contaminants have a high H, then direct toxicity will be the most important exposure pathway, followed by biomagnification.
For rapidly degrading contaminants (that is, t1/2 = fast), the metabolites of the contaminant might have a larger impact on the environment than the parent contaminant. Therefore, it is necessary to assess the toxicity of the parent contaminant and to separately assess the toxicity and exposure pathways of the metabolites, as these can be markedly different from the parent contaminant. It would be preferable for metabolites to have their own EIL and/or SQG values. However, in practice, the number of EILs and/or SQGs for metabolites will be very limited due to a lack of knowledge of their toxicity and environmental fate.
Table 4. The properties (half-life t½; logarithm of the octanolwater partition coefficient log KOW; Henry’s gas law constant H) used to assess the importance of the various exposure pathways for organic contaminants and the corresponding two most important routes
t½a
| Log Kowb
| H b
| Exposure routes to be considered | |
Primary | Secondary | |||
S | H | LM | Biomagnification | Direct toxicity |
S | H | H | Direct toxicity | Biomagnification |
S | L | LM | Direct toxicity | Metabolites |
S | L | H | Direct toxicity | Metabolites |
M or F | H | LM | Direct toxicity | Metabolites |
M or F | H | H | Direct toxicity | Metabolites |
M or F | L | LM | Direct toxicity | Metabolites |
M or F | L | H | Direct toxicity | Metabolites |
a. S = slow, M = moderately fast, F = fast. b. H = high, M = medium, L = low
There is no straightforward physicochemical property of inorganics that will predict their biomagnification potential, unlike organic contaminants. In the past, the bioconcentration, bioaccumulation and biomagnification factors (BCF, BAF and BMF respectively) have been used for this purpose, but this is not appropriate (Luoma & Rainbow 2008). Unless there is clear evidence that an inorganic element does not biomagnify, it should be considered to biomagnify and therefore secondary poisoning should be considered when deriving the EIL and/or SQG for that contaminant. A preliminary list of inorganic elements that do and do not biomagnify is given in Table 5 below.
Table 5. A preliminary list of inorganics known to biomagnify or known to not biomagnify based on information in the literature.
Biomagnification status | Inorganic contaminants |
Known to biomagnify | Cd, Hg (especially methyl forms), Se |
Known to not biomagnify | As, Cu, Fe, Mg, Pb, Zn |
Only three biomagnification classes for inorganics should be used: known biomagnifiers, known non-biomagnifiers, and unknown biomagnifiers (which are then treated as biomagnifiers pending further investigation).
Henry’s law constant (H) is a measure of the volatility of the element, as described previously. Inorganic elements and contaminants in general have very low volatility. Therefore, exposure pathways involving volatility should only be considered for mercury. These have not been included in the method used to determine the important exposure routes for inorganics.
Table 6 below presents the two exposure routes for inorganic contaminants that are considered the most important for deriving EILs and/or SQGs, depending on whether the contaminant biomagnifies or not.
For unknown and known biomagnifying inorganics, secondary poisoning should be addressed. For all inorganic contaminants, direct toxicity to relevant species and soil processes should be addressed.
Table 6. The property used to conduct the inorganic contaminant exposure pathway assessment with the corresponding two most important exposure routes
Biomagnifies
| Exposure routes to be considered | |
Primary | Secondary | |
Yes | Biomagnification | Direct toxicity |
No | Direct toxicity | |
Unknown | Biomagnification | Direct toxicity |
A schematic of the methodology to derive EILs for contaminants is given in Figure 2 below. The main steps in the methodology are:
EIL = ABC + ACL (equation 1)
The separation of naturally occurring concentrations of a contaminant and the added contaminant in deriving EILs and/or SQG is based on the ‘added risk approach’ (Struijs et al. 1997; Crommentuijn et al. 1997). This approach assumes that the availability of the ABC of a contaminant is zero or sufficiently close that it makes no practical difference. But, more importantly, it assumes that the background ‘has resulted in the biodiversity of ecosystems or serves to fulfil the needs for micronutrients for the organisms in the environment’ (Traas 2001). Therefore, the approach views only the effect of added contaminants to the environment as adverse. This approach is mostly relevant for ecological risk assessment (ERA) but less relevant for human risk assessment.
Evidence supporting the assumptions of the added risk approach has been provided by Posthuma (1997) and Crommentuijn et al. (2000b) and by work showing that the availability of metal salts decreases over time through aging processes (Posthuma 1997; Song et al. 2006). However, for microbial communities the background might be important regarding the development of tolerance to the metals (Díaz-Raviña & Bååth 1996; Bååth et al. 1998; Rutgers et al. 1998; McLaughlin & Smolders 2001; Rusk et al. 2004; Fait et al. 2006; Broos et al. 2007). Some of these studies found positive relationships between metal background concentration and effect concentrations, which could indicate that microbial communities in soils with relatively high background metals have evolved to be more tolerant to additional metal. Although these studies have shown that background concentration might not be completely inactive, adaptation of microbial communities does not lead to an underestimation of the ACL; rather, it is more likely to cause overprotection for microorganisms.
The first step in the methodology of deriving an EIL and/or SQG is to conduct a literature review and/or to search databases, such as the US EPA ECOTOX database (US EPA 2004), Australasian ecotoxicology database[5] (Warne et al. 1998; Warne & Westbury, 1999; Markich et al. 2002; Langdon et al. 2009) or the ECETOC database (ECETOC 1993), for available toxicity data for the contaminant in question. Unlike the situation in the derivation of HILs, it is not appropriate to have a hierarchy of data sources to be used in deriving EILs and/or SQGs. For most metals and well-known organic contaminants, toxicity data in addition to that found in the above databases will be available in the literature. Therefore, one should not rely solely on these databases.
For many organic contaminants there will be no toxicity data available. If there is no toxicity data available, models can be used to predict toxicity. These models include quantitative structureactivity relationships (QSARs) and quantitative activityactivity relationships (QAARs). The Australian and New Zealand WQGs (ANZECC and ARMCANZ 2000) used QSARs to derive trigger values (TVs) for narcotic organic contaminants (for example, ethanol for marine waters) when there was insufficient data. If QSARs or QAARs are not available, the equilibrium partitioning method (Van Gestel 1992; ECB 2003) can be used if toxicity data is available for aquatic species.
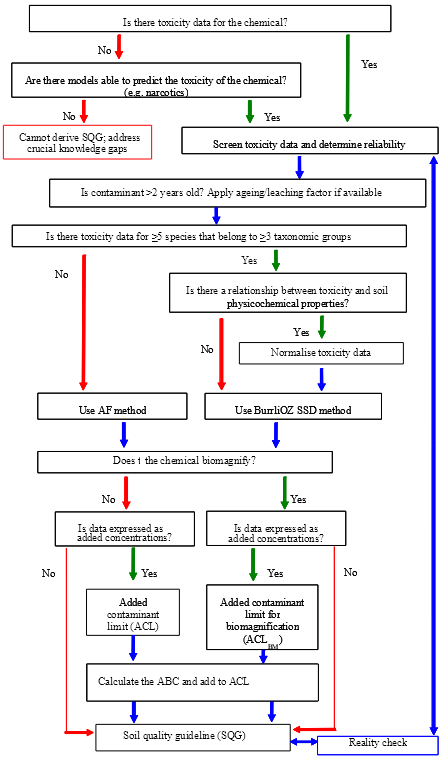
Figure 2. Schematic of the methodology for deriving ecological investigation levels (EILs) for Australian soils.
QSARs are empirical relationships between the toxicity of contaminants to a particular test organism and one or more physicochemical properties of the contaminant. QSARs are derived for contaminants with either the same mechanism of action or similar contaminant structures. The most widely used physicochemical property is log Kow. An example of a typical QSAR is presented below:
log EC 50 = -0.72 log Kow + 3.37 (equation 2)
where log EC50 (μmol/L) is the concentration at which 50% growth inhibition of lettuce (Lactuca sativa) was observed (Hulzebos et al. 1991).
The toxicity of contaminants with the same mechanism of action or chemical structure as those in the QSAR can be predicted based on their physicochemical properties. The prediction is made by substituting the value of the contaminant into the QSAR. If equation 2 was being used, the log Kow of a contaminant would be substituted into the equation.
QSARs have been developed for terrestrial plants (Hulzebos et al. 1991) and invertebrates (Van Gestel et al. 1991); however, they are not as widely available as for aquatic species (Posthumus & Slooff 2001). Only QSARs derived using terrestrial species should be used to derive EILs and other SQGs.
The simplest forms of QAARs are empirical relationships that model the toxicity of contaminants with the same mechanism of action to one species using toxicity data of another species. These are termed binary relationships. An example (Westbury et al. 2004) is provided below:
log EC50 (C. d.) = 0.848 log LC50 (P. r.) + 0.047 (equation 3)
where log EC50 (C. d.) is the log of the concentration that causes a 50% immobilisation of the cladoceran Ceriodaphnia dubia, and log LC50 (P. r.) is the log of the concentration that kills 50% of the fish Poecilia reticulata.
More complex QAARs have been developed that relate the toxicity of contaminants simultaneously to multiple species (Raimondo et al. 2007; Morton et al. 2008). Both the simple and more complex QAARs allow toxicity data for one or more species to be used to estimate the toxicity to another species. Thus they can fill some of the data gaps that often occur in deriving EILs or their equivalents.
The equilibrium partitioning method (EqP) is used to predict the toxicity of a contaminant in soils based on aquatic toxicity data. The EqP is based on the assumption that the main route of exposure for soil organisms is the soil pore water concentration (Van Gestel 1992; ECB 2003). Therefore the EqP is not suitable for:
Therefore, the EqP method should only be used to assess the toxicity of the following taxonomic groups, as they meet the above criteria: annelida, bacteria, fungi, hexapoda (larvae only), nematoda, protozoa and tardigrades.
The EqP estimate of a NOEC for a contaminant in soil (NOECsoil) is calculated from the NOEC of aquatic species as indicated below:
![]() (equation 4)
(equation 4)
where RHOsoil is the bulk density of the saturated soil and Kd is the soilwater partitioning coefficient (L/kg) (ECB 2003).
While there has been work done overseas to assess the validity of the EqP method (Van Beelen et al. 2003), there has been no such work undertaken in Australia. This is not a preferred method as Australian soils are relatively old, have low concentrations of nutrients, low organic carbon contents and different clay mineralogy (Taylor 1983), and are thus quite different from European and North American soils.
The next step in the methodology is to determine the suitability of the available toxicity data. Toxicity data is considered acceptable when the:
Biomarker end points, like enzyme production, lysosomal damage and avoidance responses, are considered to be less ecologically relevant and therefore they should not be used for the derivation of EILs unless data is limited and the predictive methods discussed in the previous section are not suitable. Biomarker tests are very sensitive and are therefore considered as early warning tests. However, if such data is used to derive EILs, this should be clearly stated. Biomarker data can be highly relevant for site-specific ecological risk assessment.
Once the unsuitable toxicity data has been removed, the next step is to assess the quality of the remaining data. Such screening methods are used in the methodologies of most countries to derive environmental quality guidelines (EQGs); for example, in Denmark, the Netherlands and the USA. However, in most cases, how the data was screened is not described. A screening method was used for the Australian and New Zealand WQGs (Warne et al. 1998; Warne 2001). This method assessed whether appropriate experimental designs, chemical analyses and statistics were used to obtain the toxicity data.
The method was based on the method used within the US EPA AQUIRE database, which was later renamed the US EPA ECOTOX database (US EPA 1994, 2004) but was improved by Warne et al. (1998).
These methods were subsequently reviewed and further improved by Hobbs et al. (2005). The Hobbs et al. (2005) data quality assessment procedures were modified so they were suitable for terrestrial ecotoxicity data (see Table 7) for use in this guideline.
Table 7. Scheme to assess the quality of terrestrial ecotoxicology data. This has been modified from the aquatic scheme of Hobbs et al. (2005).
Question | Marks awarded | |
1 | Was the duration of the exposure stated (e.g. 48 or 96 hours)? | 10 or 0 |
2 | Was the biological end point (e.g. immobilisation or population growth) stated and defined (10 marks)? Award 5 marks if only the biological end point is stated. | 10, 5 or 0 |
3 | Was the biological effect stated (e.g. LC or NOEC)? | 5 or 0 |
4 | Was the biological effect quantified (e.g. 50% effect, 25% effect)? The effect for NOEC and LOEC data must be quantified. | 5 or 0 |
5 | Were appropriate controls (e.g. a no-toxicant control and/or solvent control) used? | 5 or 0 |
6 | Was each control and contaminant concentration at least duplicated? | 5 or 0 |
7 | Were test acceptability criteria stated (e.g. mortality in controls must not exceed a certain percentage) (5 marks)? or Were test acceptability criteria inferred (e.g. test method used (US EPA, OECD, ASTM, etc.)) (award 2 marks). Note: Invalid data must not be included in the database. | 5, 2 or 0 |
8 | Were the characteristics of the test organism (e.g. length, mass, age) stated? | 5 or 0 |
9 | Was the type of test media used stated? | 5 or 0 |
10 | Were the contaminant concentrations measured? | 4 or 0 |
11 | Were parallel reference toxicant toxicity tests conducted? | 4 or 0 |
12 | Was there a concentration–response relationship either observable or stated? | 4 or 0 |
13 | Was an appropriate statistical method or model used to determine the toxicity? | 4 or 0 |
14 | For NOEC/LOEC data, was the significance level 0.05 or less? or For LC/EC/BEC data, was an estimate of variability provided? | 4 or 0
|
15 | Were the following parameters measured and stated? (3 marks if measured and stated, 1 if just measured) pH OM or OC content clay content CEC |
3, 1 or 0 3, 1 or 0 3, 1 or 0 3, 1 or 0 |
16
| Was the temperature measured and stated? | 3 or 0 |
17 | Was the grade or purity of the test contaminant stated? | 3 or 0 |
18 | Were other cations and/or major soil elements measured? or Were known interacting elements on bioavailability measured (e.g. Mo for Cu and Cl for Cd)? | 3 or 0 |
19 | For spiked soils with metal salts: were the soils leached after spiking? | 3 or 0 |
20 | Were the incubation conditions and duration stated? | 3, 1 or 0 |
| Total score Total possible score for the various types of data and contaminants: 102 |
|
| Quality score (%) (Total score /102 * 100) |
|
| Quality class (H ≥80%, A 51%–79%, U ≤ 50%)a |
|
a H = high quality, A = acceptable quality and U = unacceptable quality.
Each experimentally derived toxicity datum should have its quality assessed by the data quality assessment scheme (Table 7), which asks 20 questions, with marks awarded depending on the answer to the questions. The quality score for each datum is determined by expressing the total score obtained as a percentage of the maximum possible score. The toxicity data is then classified into three classes depending on the quality score. Data with a quality score ≤50%, between 51% and 79% and ≥80% were classed as unacceptable (U), acceptable (A), and high (H) quality respectively. Only acceptable and high quality data should be used to derive EILs.
Only toxicity data expressed as either added or total soil concentrations should be used to derive EILs. There is considerable evidence both from overseas (Smolders et al. 2003; Smolders et al. 2004; Oorts et al. 2006; Zhao et al. 2006) and within Australia (Broos et al. 2007; Warne et al. 2008b) that chemical extract concentrations; for example, calcium chloride, ammonium nitrate and soil solution extracts, are not necessarily better measures of bioavailability than total concentrations for inorganic contaminants where contamination occurred in soluble forms. Furthermore, there is also considerably more toxicity data expressed as total metal concentration, and there is regulatory acceptance and understanding of this concentration measure.
By this point in the methodology, the available toxicity data has been collated or models used to derive estimates and the data has been assessed for its appropriateness and quality. The obtained data requires standardisation in terms of four factors:
Please note that this is not the normalisation step that accounts for the effect that soil characteristics have on toxicity values.
There are many different measures of toxicity. The most frequently used toxicity measures to derive EQGs are NOECs and EC/LC50-type data. However, not all studies report these particular measures of toxicity; for example, the toxicity may be reported as an EC25 or an LC40. Therefore, in order to maximise the data available to derive EILs, it may be necessary to estimate the reported toxic effect.
A number of studies (Moore & Caux 1997; US EPA 1991; Hoekstra &Van Ewijk 1993) have shown that NOECs, while not statistically different from the control, typically correspond to a 1030% effect, with 75% of NOECs corresponding to less than a 20% effect (Moore & Caux 1997). LOEC values would of necessity cause higher percentage effects and have a median of 30% (Moore & Caux 1997). For the purposes of this methodology, toxicity data that caused less than a 20% effect; for example, EC0 to ≤EC19, are considered equivalent to NOEC data and for brevity are referred to as NOEC and EC10 data. Toxicity data that cause a 2040% effect are considered equivalent to LOEC data and are referred to throughout this guideline as LOEC and EC30 data. Toxicity data that cause >4060% effect are considered equivalent to EC50 data and are referred to as EC50 data.
Due to the general paucity of terrestrial ecotoxicology data, if toxicity data is not expressed as a single value but instead is given as ranges, then the lowest value of the range should be used in order to provide a conservative estimate of the toxicity. In certain studies, the lowest toxicant concentration had already caused significant toxic effects and therefore toxicity data are given as a < or ≤ value. If possible, the percentage effect that the reported concentration caused should be determined and, using the ranges stated in the previous paragraph, be considered equivalent to NOEC, LOEC or EC50 data, and they should be converted accordingly. Toxicity with an effect greater than 60% should not be used to derive EILs. If, in studies, the highest tested concentration did not cause an effect or a statistically significant effect on the test species (that is, an unbounded NOEC), then the toxicity data should be given a > value and treated as an EC10. This is done as it is a conservative approach and will result in more toxicity data available for EIL and/or SQG derivation.
As stated earlier, EILs are to be derived using LOEC and EC30 toxicity data. But such data is not always generated in toxicity studies. Therefore, in order to maximise the data available to derive EILs, toxicity data can be converted to LOEC and EC30 data. Two different approaches were applied to the different measures of toxicity data in the Australian and New Zealand WQGs (ANZECC & ARMCANZ 2000). For organics, only chronic NOEC data was considered acceptable to derive high reliability TVs, while only acute EC/LC50 values were suitable for moderate reliability TVs and either NOEC or EC/LC50 data was suitable for low reliability TVs (Warne 2001). In contrast, for metals, chronic NOEC, LOEC, EC/LC50 and maximum acceptable toxicant concentrations (MATC) values could be used provided all non-NOEC values were converted to NOEC values (Warne 2001). This was done using a series of default conversion factors (see Table 8 below). The reason for the different approaches was that for the organic contaminants, generally the chronic data was NOEC values, whereas the vast majority of the chronic metal toxicity data was EC/LC50 values (Warne 2001).
Table 8. Default conversion factors used to convert different chronic measures of toxicity to chronic NOECs in the Australian and New Zealand WQGs (ANZECC & ARMCANZ 2000). Values are from Warne (2001).
Toxicity dataa | Conversion factor |
EC50 to NOEC or EC10 | 5 |
LOEC or EC30 to NOEC or EC10 | 2.5 |
MATC* to NOEC or EC10 | 2 |
a EC50, EC30 and EC10 values are the concentrations that cause a 50%, 30% or 10% effect, NOEC = the no observed effect concentration, LOEC = lowest observed effect concentration, MATC = the maximum acceptable toxicant concentration and is the geometric mean of the NOEC and LOEC.
The more flexible method that was applied to the metals in the Australian and New Zealand WQGs (ANZECC & ARMCANZ 2000) and the conversion factors that were used (see Table 8) were used in the EIL derivation methodology. It should be noted that these conversion factors are based on expert judgement (Warne pers. comm.). Therefore, if sufficient terrestrial data is available to derive terrestrial conversion factors then these should be used. For example, data from the Australian National Biosolids Research Program indicates that the phytotoxicity chronic EC10 to chronic EC50 conversion factor for cations such as Cu and Zn was 3 (unpublished data).
Compared to aquatic toxicity studies, there is a limited number of terrestrial toxicity studies. Therefore, maximum use must be made of the available toxicity data and data should be converted from one measure to another (see above).
However, if more data become available then it should be used in the following descending order of preference:
There are a number of well-acknowledged limitations to NOEC and LOEC data (Newman 2008; Fox 2008; Warne & Van Dam 2008). Some scientists (Chapman et al. 1996) have argued that they should not be used to derive EQGs. However, they continue to be used for that purpose because no regulatory authority has recommended an alternative measure of toxicity be used and because a large amount of this type of data is available. For these reasons, the Australian and New Zealand WQGs (ANZECC & ARMCANZ 2000) used NOEC data but suggested that the use of NOEC data ’be phased out‘ as EC10-type data become available. Warne and Van Dam (2008) have gone one step further by calling for a ban on the generation and use of NOEC and LOEC data in Australia. Since the Australian and New Zealand WQGs were published, more researchers are reporting EC/LC10 to EC/LC20-type toxicity data. The use of point estimate toxicity data is therefore preferred.
The EIL derivation methodology makes a clear distinction between natural background concentration, which is the natural level of contaminants in the soil, and ABC, naturally occurring background and the contaminant levels that have been introduced from diffuse or non-point sources by general anthropogenic activity not attributed to industrial, commercial, or agricultural activities. Therefore, it is preferable that all toxicity data is expressed as an added concentration. If the toxicity data is not expressed in terms of added contaminant then they should be converted to that form, if possible. This can be achieved by subtracting either the ABC, if it is known, or the average concentration in the control soil (that is, the test soil with no addition of the test contaminant) from the total concentrations and then re-calculating the toxicity. If background concentrations are not given then, for some inorganics, the method of Hamon et al. (2004) can be used to estimate ABC in Australian soils or the Dutch background correction equations (Lexmond et al. 1986) can be used to estimate the background concentration. Alternatively, one can set a default background level or assume that the background concentration was zero.
The Australian and New Zealand WQGs (ANZECC & ARMCANZ 2000) make a clear distinction between chronic and acute toxicity data and convert TVs derived using acute EC/LC-type data to chronic TVs by using, in order of decreasing preference, acute to chronic ratios (ACRs) or a default AF of 10. This approach is very common and widely used in water quality guidelines (ANZECC & ARMCANZ 2000; CCME 1991; US EPA 1991) but is not used in soil guidelines. This is due mostly to the fact that the exposure duration of most terrestrial ecotoxicity tests is three to four weeks. Therefore, conversion factors should only be used for short-term exposure tests. If ACR values are available then they should be used to convert acute terrestrial toxicity data. Only if ACR values are not available should a default AF of 10 be used, which is consistent with the approach adopted by the Australian and New Zealand WQGs (ANZECC & ARMCANZ 2000).
In deriving any EQGs, the question always arises as to whether toxicity data for overseas species should be used. By using toxicity data for overseas species, the assumption is made that they have the same sensitivity as endemic species. The validity of this assumption has been questioned and examined in a number of studies using aquatic species (Dyer et al. 1997; Markich & Camilleri 1997; Brix et al. 2001; Hobbs et al. 2004; Hose & Van den Brink 2004; Maltby et al. 2005; Chapman et al. 2006; Kwok et al. 2007). However, the evidence is conflicting, with some studies (Maltby et al. 2005; Hose & Van den Brink 2004) finding no differences while others have found differences (Dyer et al. 1997; Markich & Camilleri 1997; Brix et al. 2001; Hobbs et al. 2004; Chapman et al. 2006; Kwok et al. 2007). Kwok et al. (2007) combined results from SSD analysis with ERA principles to determine that, in order to protect 95% of tropical aquatic species, toxicity data for temperate aquatic species should be divided by a factor of 10. Using a similar methodology, Hobbs (2006) found that if Australasian species were to be protected from 95% of chemicals, then toxicity data for northern hemisphere freshwater and marine/estuarine species would have to be divided by 6.2 and 2.2 respectively. The inconsistency in the published results led Chapman et al. (2006) to conclude that ’toxicity data from one geographic region will not be universally protective of other regions‘.
The other factor that needs to be considered in resolving this issue is that from a statistical point of view EILs and/or SQGs become increasingly reliable as the number of species for which there is toxicity data increases. Therefore, as a pragmatic compromise, toxicity data for both endemic and overseas species should be used to derive EILs. This is consistent with the Australian and New Zealand WQGs (ANZECC & ARMCANZ 2000). However, if there are four or more toxicity data measurements in Australia for a species; that is, they meet the minimum data requirements to derive EILs and SQGs, then this should be used in preference to toxicity data for the same species tested overseas.
Typically, soil toxicity tests use soils that have been freshly spiked with the contaminant in question. There are very limited amounts of toxicity data available for soils where the contaminant was added some time prior to testing, let alone field-aged soils contaminated by a variety of sources of contaminants with varying bioavailability. The predominance of laboratory-spiked toxicity data has implications for the derivation of EILs due to ageing and leaching.
Ageing or natural immobilisation (attenuation) is the process by which many contaminants (both inorganic and organic), when added to soil, will bind over time to various soil components (Barrow 1986; Hamon et al. 2007; Smolders & Degryse 2007) and this can reduce the concentration of the contaminant that is biologically available (McLaughlin et al. 2000a). Leaching is a process that removes readily soluble soil components such as salinity from soils. Most laboratory-spiked toxicity tests do not leach the soils after the spiking and this has the effect of increasing the ionic strength, decreasing soil pH, increasing aqueous concentrations of dissolved cations (such as Ca, Mg, K, Cd, Cu, Ni, Pb, etc.) and anions (Cl, SO4, NO3, etc.), and ultimately increasing the toxicity (Stevens et al. 2003). A study by Oorts et al. (2006) examined the magnitude of the ageing and leaching effects on the toxicity of Cu and concluded that leaching accounts for the majority of the observed difference in toxicity between freshly spiked and aged soils. A study by Smolders et al. (2009), the findings of which have been incorporated into the Flemish SQGs (VLAREBO 2008), derived ageing/leaching factors (ALFs) for Zn2+ (3), Cu2+ (2), Ni2+ (13), Co2+ (1.13.5), Pb2+ (4.2), Cd2+ (1) based on toxicity measures in a variety of European field and freshly spiked soils.
This is the only study that has generated such ALFs across a wide range of soils and ecotoxicity end points. These ALFs were developed based on a maximum of 18 months ageing and leaching (Smolders et al. 2009). These ALFs should be used in deriving EILs when the contaminants have been present in the soil for at least 2 years. This would be achieved by multiplying the non-aged and non-leached toxicity data by the appropriate ageing/leaching factor, thus decreasing their ’effective‘ toxicity. Thus, EILs for both fresh (contaminants have been in the soil for less than 2 years) and aged (the contaminants have been in the soil for greater than 2 years) contamination can be derived.
Currently, there are very few ALFs available, particularly for Australian soils. There are no ALFs for organic chemicals. When ALFs are not available, it is not possible to derive EILs for aged contamination. In such cases, there are two potential approaches. Firstly, conduct research to derive ALFs for the contaminant of concern or, secondly, conduct direct toxicity assessments (DTA) using soil from the site under investigation. If sufficient toxicity tests are conducted, then site-specific EILs could be derived in much the same manner as deriving site-specific WQGs (ANZECC & ARMCANZ 2000).
There are two potential methods that can be used to derive ACLs: the AF method — a worst-case scenario approach, and the SSD method — a risk-based approach. Both approaches require a minimum amount of toxicity data to derive EILs. The preferred methodology to calculate EILs is the SSD approach because this is a risk-based approach. However, which method is used to derive EILs depends on the number of species and taxonomic groups for which there are toxicity data (see Table 9 below).
Unlike the toxicity data for terrestrial species, toxicity data for soil processes is not based on single species but rather a community of microbial species that perform that soil process. Thus, strictly speaking, it is not suitable for use in SSD methods. However, these processes are important measures of soil ecosystem health and should be protected. The preferred method for deriving EILs is therefore to use the normal single species toxicity data but also soil process toxicity data.
SSD methods require a minimum set of toxicity data for the aquatic environment, which is usually specified in terms of a minimum number of species and taxonomic groups for which data is required. However, such an approach is not suitable for soil processes where the desirable data types are the number of soil processes and the number of nutrient groups.
A nutrient group is considered to be all toxicity end points measured that relate to a particular nutrient (see Table 11 below). For example, toxicity data for substrate-induced nitrification, potential nitrification rate and denitrification would all belong to the nitrogen nutrient group.
As the number of species and taxonomic groups or soil processes and nutrient groups for which toxicity data is available decreases, the confidence that the resulting EIL will provide the desired level of protection also decreases. In an attempt to compensate for this, the percentage of species and/or soil processes to be protected by the EILs increases as the number of species or soil processes and taxonomic groups or nutrient groups for which toxicity data is available decreases (see Table 9 below).
Table 9. Number of species or functional processes and number of taxonomic groups or nutrient groups needed for the SSD and AF approaches and the corresponding level of protection provided for residential land. The same principle of increasing the level of protection as the amount of toxicity data decreases also applies to other soil quality guidelines and for other land uses (i.e. the default level of protection would increase by 5% if there was data for 5 to 8 species or functional processes)
Number of species or functional processes | Number of taxonomic or nutrient groups | Methodology to derive EIL | Percentage of species to be protected |
≥9 | ≥3 | SSD Burr III | 80% a |
58 | ≥3 | SSD Burr III | 85% a |
38 | <3 | AF | Not relevantb |
a add 5% to the percentage of the species or soil processes to be protected if the contaminant is a biomagnifier.
b The AF does not determine EILs based on protecting a certain percentage of species.
The decision by regulatory agencies about the minimum data requirements is often arbitrary (Pennington 2003) and is based on pragmatic considerations. The US EPA requires at least eight species (US EPA 1999), the Dutch suggests ten species for EQGs (van Vlaardingen & Verbruggen 2007) although some studies have used five species (Van de Plassche et al. 1993; ANZECC & ARMCANZ 2000) and four species (Crommentuijn 2000a), and between five and eight species (OECD 1992, 1994). Since 2000, a number of publications have shown the importance of having larger data sets. For example, Newman et al. (2000) used non-parametric methods to estimate for 30 toxicants that approximately 15 to 55 (with a median of 30) species were needed per toxicant in order produce reliable EQGs. In another example, Wheeler et al. (2002) estimated that a minimum of 10 to 15 species per toxicant are needed. Subsequently, the European Union (EU) has recommended in the technical guidance document on aquatic risk assessment (ECB 2003) that the minimum toxicity data requirement is ten species that belong to eight taxonomic groups. Thus, while it is preferable to use toxicity data sets containing more species and taxonomic groups (or more soil processes and nutrient groups), this must be weighed against the fact that for soil and terrestrial ecosystems there is a general lack of toxicity data. If it were decided to use the same minimum data requirements as the EU, then EILs could be derived for only a limited amount of contaminants using the preferred SSD method. Other contaminants would have to be derived using the second choice AF method, likely to generate highly conservative criteria. It is imperative to acknowledge the situation for terrestrial systems and to set reasonable minimum data requirements for the SSD method, in order that the majority of the EILs are derived by the preferred SSD method.
Studies by the Danish EPA (Pedersen et al. 1994) and the OECD (1995) indicated that WQGs derived using data sets containing less than five values were very dependent on the spread of the values, whereas for data sets containing five or more values, this effect was markedly reduced. Therefore, the recommended minimum number of species and/or soil processes required to use the SSD approach is five. The minimum number of taxonomic or nutrient groups for toxicity data required in order to use the SSD method was reduced to three. Between five and eight species and/or soil processes, the SSD approach still has a large variation and uncertainty and therefore the protection level should be increased by 5% of species and/or soil processes in order to be more certain that the desired level of protection is achieved. If toxicity data for more than eight species and/or soil processes is available, the SSD approach is deemed to be sufficiently robust to set the protection limit for the appropriate land use (Table 9 above).
In order to determine which method (either the SSD method or the AF method) can be used to derive the EIL, the screened toxicity data should firstly be grouped together on the basis of species or soil processes. Then, using the information presented in Tables 10 and 11 below, the number of taxonomic groups and/or nutrient groups for which toxicity data is available can be determined.
If there is sufficient terrestrial toxicity data for a contaminant, toxicity data derived by models like QSARs or QAARs and the equilibrium partitioning approach should not be used. However, if there is insufficient terrestrial toxicity data available to meet the SSD requirements, the modelled data should be used in combination with measured toxicity data. The minimum data requirements to use the SSD and AF methods are the same when using a data set containing both measured and modelled toxicity data as when using only measured toxicity data. However, only low reliability EILs can be generated using modelled toxicity data (Section 2.4.11).
Table 10. The taxonomic groups for terrestrial species
Taxonomic group | Examples of species in this group |
Mollusca | Snails, slugs |
Annelida | Enchytraeids, earthworms |
Nematoda | Nematodes |
Hexapoda | Insects, springtails |
Myriapoda | Centipedes, millipedes |
Chelicerata | Mites, spiders |
Crustaceans | Woodlice |
Algae | Algae |
Plantae | Plants |
Fungi | Fungi |
Bacteria | Bacteria |
Protozoa | Amoebas, ciliates, flagellates |
Tardigrada | Water bears |
Chordata | Reptiles, mammals, birds |
Table 11. The nutrient groups for soil (i.e. microbial and fungal) processes
Nutrient group | Soil process | Examples of end points |
C cycle | Aerobic decomposition | Basal respiration, substrate-induced respiration |
N cycle | N mineralisation/ammonification | Urease activity, NH4 production |
| Nitrification | NO3 production, substrate-induced respiration |
| Denitrification | Nitrate reductase |
| Nitrogen fixation | Nitrogenase activity |
P cycle | P mineralisation | Phosphatase, Py-phosphatase |
S cycle | S mineralisation | Aryl-sulfatase |
The SSD approach is a statistical method to calculate a soil concentration that theoretically protects a specified percentage of species and/or soil processes. The SSD method used to derive the Australian and New Zealand WQGs (ANZECC & ARMCANZ 2000) was the Burr Type III method (Shao 2000), which was incorporated into the BurrliOZ program (Campbell et al. 2000) that is available from: www.cmis.csiro.au/Envir/burrlioz/
Download1.htm.
If there are screened toxicity data values for a contaminant to at least five species or soil processes for three taxonomic or nutrient groups, then there is sufficient data to calculate an ACL using the Burr Type III SSD method.
All SSD methods use a single numerical value to describe each species or soil process for which toxicity data is available. The means by which a single value was obtained for each species or soil process (Van de Plassche et al. 1993) are set out below:
SSD methods require the toxicity data to have a uni-modal distribution. If the data set is not uni-modal (for example, insecticides are more toxic to insects than mammals), then the toxicity data belonging to the most sensitive distribution should be used for ACL derivation, as recommended by Warne (1998, 2001) when deriving WQGs.
The use of normalisation relationships is an attempt to minimise the effect of soil characteristics on the toxicity data so the resulting toxicity data will more closely reflect the inherent sensitivity of the test species to the contaminant. If toxicity data more closely reflects species sensitivity, then a more accurate calculation of the soil concentration that should protect a certain percentage of species and soil processes can be made. Derivation of soil-specific EILs and the use of normalisation relationships to normalise toxicity data can only be done if there is sufficient data to use the SSD method. Toxicity data should not be normalised if the available toxicity data is only sufficient to meet the minimum data requirements of the AF approach.
If the toxicity data for a contaminant has been demonstrated to be affected by soil characteristics, (that is, by statistically significant (p ≤ 0.05) normalisation relationships between toxicity data and soil characteristics), then the toxicity data must be normalised to the Australian reference soil (see Table 12 below).
Table 12. Values of soil characteristics for the Australian reference soil to be used to normalise toxicity data
Soil property | Value |
pH: | 6 |
Clay: | 10% |
CEC: | 10 cmol/kg |
Org. Carbon: | 1% or equivalent OM |
Normalisation relationships are currently limited to a few combinations of contaminants, species and countries from which the soils are obtained (Smolders et al. 2004; Li et al. 2003; McLaughlin et al. 2006; Song et al. 2006; Broos et al. 2007; Warne et al. 2008a; 2008b). This is predominantly due to the concept of developing normalisation equations for terrestrial ecotoxicity data being relatively recent and the size of, and cost of conducting, such work.
The lack of normalisation equations for a wide variety of species can be overcome by applying the relationships across species within the following groupings of the taxonomic groups:
These groupings are based on the basic body design of the organisms and the likely exposure route of organisms to the contaminant; that is, being exposed by the direct environment or through food. The following four derivation steps are listed in order of descending order of preference:
The above steps are used to standardise the derivation of realistic EILs that are protective but at the same time ensure that the EILs do not become too conservative.
If the toxicity data shows a significant relationship with specific soil characteristics; for example, soil pH, organic carbon or clay content, cation exchange capacity (CEC), soil-specific ACL values can be calculated using those relationships. The toxicity data is first normalised to the reference Australian soil using the methods described above, and the ACL value derived using the SSD approach is valid for the Australian reference soil. Using the normalisation relationships, ACL values can then be calculated for different soil types. For example, if toxicity data showed a relationship with pH, different ACL values can be calculated for a range of soil pH conditions.
The lack of normalisation equations for soils from Australia can be overcome by using normalisation relationships developed with soils from other countries, particularly Europe and America. However, these normalisation relationships should only be used when they are derived from soils similar to Australian soils and/or their validity for Australian soils has been assessed and found suitable. The importance of this was shown by a study of Broos et al. (2007), which assessed the normalisation relationships of Smolders et al. (2004) and Oorts et al. (2006) for microbial nitrification in soils. They re-analysed the overseas data after removing microbial toxicity data for soils with organic compound concentrations greater than those found in Australian soils. This resulted in a change of soil characteristics, explaining the variance in the toxicity data.
A second option to overcome the lack of normalisation relationships in the literature is to examine the currently available toxicity data and use regression analyses on the collated data to determine if a significant relationship exists between toxicity and soil characteristics.
Normalisation relationships from field studies are preferred over those from laboratory studies. All the normalisation relationships for toxicity, apart from those developed by Broos et al. (2007) and Warne et al. (2008b), model laboratory-based data (Rooney et al. 2006; Smolders et al. 2003; Smolders et al. 2004; Oorts et al. 2006; EU 2006b; Song et al 2006, Warne et al 2008a). Warne et al. (2008b) found that field-based normalisation relationships gave much more accurate estimates of field phytotoxicity than laboratory-based normalisation equations. Therefore, field-based normalisation relationships should be used in preference to laboratory-based normalisation relationships. It is, however, realised that the current lack of field-based normalisation relationships will unavoidably necessitate the use of laboratory-based relationships, despite their limitations.
If multiple normalisation relationships are available within a taxonomic group of organisms, then the most geographically appropriate normalisation relationship should be applied to the toxicity data. For example, a European normalisation relationship would be applied to European data and an Australian normalisation relationship would be applied to Australian data. If there are multiple geographically appropriate normalisation relationships for a group of organisms, then the relationship with the lowest slope should be used, as this will give the most conservative normalised toxicity data (EC 2008).
If the minimum data requirements for the SSD approach cannot be met, the AF approach should be used to derive EILs. The AF is a ‘worst-case scenario’ type of approach. In this approach the lowest toxicity value for a contaminant; that is, the most sensitive data point, is divided by an AF in order to derive an ACL.
![]() (equation 5)
(equation 5)
Equation 5 applies to the derivation of EILs; if other SQGs were to be derived, then different toxicity data would be substituted in the equation. The magnitudes of the AFs depend on the available toxicity data and are given in Table 13 below. If there is toxicity data for less than three species, the AF is 500, due to the lack of information and thereby the high uncertainty in estimating the risk posed by the contaminant in the soil. If there is toxicity data for more than three species the AF decreases, depending on how many taxonomic or nutrient groups are represented (see Tables 10 and 11 above for taxonomic and nutrient groups respectively). If field data or model ecosystems with multiple species tested are available, an assessment has to be made as to how well the study represents the field situation and how protective the toxicity data is. An AF of 10 should be used if the EIL is calculated using mesocosm or microcosm data.
Table 13. Assessment factors to be used to derive ACL using the AF approach (adapted from ANZECC & ARMCANZ 2000).
Toxicity data available for derivation of ACL | ||
Number of species | Number of taxonomic or nutrient groups | Assessment factor |
<3 species | N/A | 500 |
≥3 species | 1 | 100 |
2 | 50 | |
<5 species | 3 | 10 |
Field data/data of model ecosystems |
| 10 |
N/A = not applicable
Secondary poisoning can occur if contaminants accumulate from the ambient environment (for example, soil) into the tissue of organisms (bioaccumulation) that are then consumed by other organisms and the concentration in tissue increases in the journey up the food chain (for example, soil, earthworms, birds and predatory birds). In such a situation, the species at most risk are the species higher in the food web (the predators). Examples of contaminants that biomagnify and have shown adverse effects on predators include DDT, cadmium and PCBs (Morrissey et al. 2005; Jongbloed et al. 1996; Luoma & Rainbow 2008). Biomagnification and secondary poisoning should only be addressed for contaminants that show biomagnification potential.
Secondary poisoning should be addressed for residential EILs. Residential areas cover a large area and can harbour many birds and small land species that can potentially be at risk from contaminants that biomagnify. For site-specific risk assessment, secondary poisoning EILs may not be relevant for contaminated sites of limited area.
The vast majority of ecotoxicological data is derived from direct exposure from the ambient environment and not from food. Thus, if a contaminant biomagnifies, then normal toxicity data and EILs derived using such data may underestimate the impact the contaminant has on the environment and communities. Therefore, a more protective measure is needed for biomagnifying contaminants.
If an SSD approach were used to derive the EIL for contaminants that biomagnify, the level of protection (that is, percentage of species and/or soil processes to be protected) should be increased by 5%, i.e. to 85% (or to 90% if <8 taxonomic species or functional processes are used). This approach is consistent with that used in the Australian and New Zealand WQGs (ANZECC & ARMCANZ 2000) to deal with secondary poisoning.
If the EIL were derived using the AF approach, then a BMF will have to be applied in order for the EIL to account for biomagnification.
The ACL for biomagnification will be calculated by:
![]() (equation 6)
(equation 6)
If there is sufficient BMF data available for an organic contaminant, then the 80th percentile of these values should be used in equation 6 above. For those organic contaminants that have no BMF values, BMF values for organic contaminants with similar chemical structures should be collated and then a specific percentile value could be adopted. The percentile of BMF values to be used is set at 80%.
For inorganic contaminants, grouping of BMF values is not recommended and biomagnification should be dealt with on an individual chemical basis.
For organic contaminants, the BMF values depend on the Kow of the contaminant and increase to 10 for organic contaminants having a log Kow of 5–8. For inorganic contaminants, the Kow values of the contaminant should not be used but the literature should be searched for BAF or BMF for terrestrial species, or fish if no terrestrial data is available. If BMF values are not available for an inorganic contaminant or a group of organic chemicals, a conservative biomagnification factor should be used. The biomagnification factors for organic contaminants, from the European technical guidance for risk assessment (ECB 2003), which are shown in Table 14 below, should be used.
Table 14. Default BMF values for organic substances that correspond to the logarithm of the octanolwater coefficients and the BCFs adapted from ECB (2003).
log Kow of contaminant | BCF (fish) | BMF |
<4.0 | <2,000 | 1 |
4.05 | 2,0005,000 | 2 |
58 | >5,000 | 10 |
>89 | 2,0005,000 | 3 |
>9 | <2,000 | 1 |
To calculate a site-specific EIL, ABCs for soils should be determined, as the ACL is based on added toxicity values. If possible, the ABCs should be directly measured at a clean reference site with a comparable soil type to the site being examined. However, such sites are not always available or easy to identify.
For metal contaminants, if reliable ABCs cannot be measured, then either the estimation method of Hamon et al. (2004) or collations of ABC values such as Olszowy et al. (1995) could be used. The equations for calculating ABC values are presented in Table 15 below. Estimates of ABCs for several metals based on example soil iron or manganese concentrations (determined by aqua regia digestion) are presented in Table 16 below. To use the Hamon et al. (2004) method, it is necessary to ascertain that the iron and manganese concentrations of the soil at the site in question are not elevated by co-contamination—these elements are normally determined in chemical analysis of soils to determine total metal concentrations and therefore minimal extra cost is involved. These Hamon et al. (2004) relationships are based on soils from sites with no known history of contamination apart from farming.
Therefore, this approach would be suitable for predicting the ABC in otherwise uncontaminated areas including new suburbs; that is, suburbs less than 20 years old (Olszowy et al. 1995). In fact, for the inorganic contaminants where comparison is possible, the ABC values predicted by the Hamon et al. (2004) method are very similar to the 25th percentile of the ABC values for new suburbs from Olszowy et al. (1995).
Olszowy et al. (1995) conducted a stratified random sampling study to determine the ABCs in residential areas of the capitals of New South Wales, Queensland, Victoria and South Australia. A total of 320 soil samples collected at 0150 mm depth were collected and analysed. If the Hamon et al. (2004) method cannot calculate an ABC, then the Olszowy et al. (1995) values for new suburbs would be appropriate to use for new suburbs or areas with no known history of contamination. In old-established urban areas (i.e. suburbs more than 20 years old), it would be appropriate to use the 25th percentile of the ABC values from Olszowy et al. (1995).
Table 15. Equations from Hamon et al. (2004) and the corresponding coefficient of determination (r2) used to estimate ABCs for arsenic (As), cobalt (Co), chromium (Cr), copper (Cu), nickel (Ni), lead (Pb) and and zinc (Zn)
Element | Normalising | Gradient | y intercept | r2 |
As | Fe | 0.547 | 0.507 | 0.50 |
Co | Mn | 0.894 | -1.409 | 0.71 |
Cr | Fe | 0.750 | 1.242 | 0.58 |
Cu | Fe | 0.612 | 0.808 | 0.61 |
Ni | Fe | 0.702 | 0.834 | 0.64 |
Pb | Fe | 1.039 | 0.118 | 0.66 |
Zn | Fe | 0.589 | 1.024 | 0.61 |
Table 16. Predicted ambient background soil concentrations (mg/kg) for arsenic (As), cobalt (Co), chromium (Cr), copper (Cu), nickel (Ni), lead (Pb) and zinc (Zn) at different soil iron concentrations, based on the equations from Hamon et al. (2004)
Soil Fe% | As (mg/kg) | Cr (mg/kg) | Cu (mg/kg) | Ni (mg/kg) | Pb (mg/kg) | Zn (mg/kg) |
0.1 | <1 | <3 | <2 | <1 | <0.1 | <3 |
1 | <3 | <17 | <6 | <7 | <1 | <11 |
10 | <12 | <98 | <26 | <34 | <14 | <41 |
20 | <18 | <165 | <40 | <56 | <29 | <62 |
Most organic contaminants of interest to contaminated sites are xenobiotics, hence they have no natural background concentration. Notable exceptions to this include lipids and fats, hormones (for example, oestrogen, testosterone), fatty acids, alcohols, hydrocarbons, polycyclic aromatic hydrocarbons (PAHs) and dioxins. Therefore, ABCs will have to be generated by direct measurement or a default ABC of zero could be assumed (Crommentuijn et al. 2000b). There are no equivalent models to that of Hamon et al. (2004) available for organic contaminants.
For dioxins, regional ABC values are available (Muller et al. 2004) and could be used or, alternatively, site-specific assessments could be conducted. For other pyrogenic organic contamination (for example, PAHs), a site-specific assessment should be conducted to determine if the measured concentrations are background concentrations for that region. If a site-specific assessment is conducted, then the upper 80th percentile of the ABCs should be used as the background as per the Australian and New Zealand WQGs (ANZECC & ARMCANZ 2000). However, even if they are considered ABCs, this does not imply that there is no risk to terrestrial biota.
If biomagnification is not considered, then the EIL for a contaminant is calculated as follows:
EIL = ABC + ACL (equation 7)
where ABC is the ambient background concentration (mg/kg) and ACL is the added contaminant limit (mg/kg).
If biomagnification is considered and is significant for that contaminant, then the EIL is calculated as follows:
EIL = ABC + ACLBM (equation 8)
where ACLBM is the contaminant added limit that accounts for biomagnification.
Classifying the EIL based on the amount and type of toxicity data is important to provide users with an indication of the reliability of the EIL values and also for prioritising future re-assessments of EILs. Methods for determining the reliability of TVs were developed and used in the Australian and New Zealand WQGs (ANZECC & ARMCANZ 2000; Warne 2001) and this formed the basis of the soil EIL reliability assessment system. The number of data points, the type of toxicity data, the number of species/soil processes for which there is data, and whether or not there are normalisation relationships are all used to assess the reliability. The three classes of EIL reliability are high, moderate and low. The requirements for an EIL to receive these classifications are provided below.
High reliability:
Moderate reliability:
Low reliability
or
In the Australian and NZ WQGs (ANZECC & ARMCANZ 2000), low reliability TVs were only used for interim guidance. A similar approach should be adopted regarding low reliability EILs—that such values should be considered to be a knowledge or data gap that requires further work to resolve.
For organic contaminants with low reliability EILs, the EILs are only as good as the QSARs and QAARs they were derived from. Therefore, further research is only necessary if the QSARs and QAARs are of relatively poor quality.
Once the EILs have been derived, their appropriateness should be evaluated. A similar process was also conducted as the last step in the derivation of the Australian and New Zealand WQGs (Warne 2001). Their appropriateness is determined by comparing each EIL with the toxicity data used to derive them, any available field-, mesocosm- or microcosm-based toxicity data, plant or crop nutritional requirements (for essential elements), and background concentrations. The aim of the comparison is to determine which species, if any, are likely to experience toxic effects if exposed to the EIL. If the species that potentially may be affected are considered rare or endangered, are keystone species, or are commercially important, then it may be appropriate to decrease the EIL (that is, increase the level of protection being provided). This evaluation or ‘ground-truthing’ process is, by necessity, done on a case-by-case basis.
A discussion of the strengths and limitations of the methodology is presented below.
The EIL derivation methodology:
The EIL derivation methodology:
In this section, the various methods used in the EIL derivation methodology are more thoroughly explained and their strengths and limitations discussed. Recommendations on which methods should be used are also provided. The methods addressed in this section are:
Soil characteristics are known to affect bioavailability and therefore the toxicity of contaminants to organisms (Lexmond 1980; McBride 1989; Alloway 1995; Basta et al. 2005). An example of the strong effects that soil characteristics have on toxicity is provided in Table 17. This shows laboratory-based toxicity data (EC10) for Cu and Zn to wheat grown in 14 different Australian soils (Warne et al. 2008a). The lowest and highest EC10 values vary 20–30 fold for both Cu and Zn. As the conditions were standardised and only one test species was used, the cause for the differences in toxicity can only be soil type and soil properties.
Table 17. Total added concentrations (mg metal/kg soil) of Cu and Zn that cause a 10% reduction in growth for wheat seedlings (EC10) grown in 14 Australian soils (Warne et al. 2008a)
Site | Cu EC10 | Zn EC10 |
Avon | 945 | 755 |
Brennans | 205 | 275 |
Bundaberg | 260 | 235 |
Cecil Plains | 3,300 | 5,855 |
Dalby | 885 | 655 |
Dookie | 490 | 965 |
Dutson Downs | - | 875 |
Esk | 465 | 565 |
Flat Paddock | 115 | 250 |
Kingaroy | 810 | 505 |
Night Paddock | 110 | 530 |
Spalding | 930 | 620 |
Tintinara | 430 | 430 |
Wilsons | 465 | 335 |
There are two methods that attempt to address the issue of the effects of soil characteristics. These are to express toxicity data in terms of a contaminant estimate of the bioavailable fraction of a contaminant and to express toxicity data in terms of total concentrations and develop relationships (termed normalisation relationships) between toxicity and soil characteristics that account for bioavailability (see McLaughlin et al. 2000a for a discussion of these two philosophies).
A number of soil extraction methods have been developed with the aim of providing a better estimate of the bioavailable fraction than total concentrations. These include calcium chloride (CaCl2) extracts, ammonium nitrate (NH4NO3) extracts, soil solution and other extracts and diffusion-based methods (for a review, see McLaughlin et al. 2000b). The extraction methods assume that they only extract that portion of the total amount of a chemical that is biologically available. This is a chemical approach to estimating the bioavailable fraction.
Available information suggests that only Germany (BBodSchV 1999) and Switzerland (Gupta et al. 1996) use a measure of chemical concentration other than the total contaminant concentration in soil. The German guidelines (BBodSchV 1999) have some soil TVs based on concentrations in NH4NO3 extracts for some inorganic contaminants (that is, TVs for cadmium) in the soil-to-plant pathway. This was only done if NH4NO3 extracts were better predictors (that is, showed better correlations) for internal plant concentrations from soil than the total soil concentration. The ammonium nitrate extract is considered by the German guidelines to be the bioavailable concentration of inorganics in soil.
The perfect chemical measure of bioavailability should give very similar toxicity values (for example, LC50) in a range of different soils for a given chemical tested on a given species. For soils, the perfect measure of bioavailability should overcome the effects that different soil characteristics have on toxicity and truly reflect the available fraction of the contaminant that causes the toxicity to the organism. Therefore, the ability of techniques to determine the bioavailable fraction can be assessed by comparing the variability of the toxicity values for one species across different soils—the measure with the smallest variability in toxicity values being the best measure of the bioavailable fraction (McLaughlin et al. 2000b). This approach was adopted by Broos et al. (2007) and Warne et al. (2008b) using microbial and plant toxicity data for Cu and Zn in 14 different Australian soils (field-based) using one source of contamination (soluble metal salts). In both cases, the variation in toxicity values based on total concentrations was smaller than or as small as those based on soil solution and CaCl2 extracts. Unpublished work from the Australian National Biosolids Research Program (NBRP) showed that the concentrations in ammonium nitrate and calcium chloride extracts were very highly related with coefficients of determination (r2) greater than 0.9. Therefore, although it is untested, it is highly likely that the data from the NBRP would reveal that variation in toxicity values across soils based on total concentrations would be lower than those based on ammonium nitrate.
A number of authors from Europe (Smolders et al. 2003; Smolders et al. 2004; Oorts et al. 2006; Zhao et al. 2006) have also found that extractable or soil solution measurements were not useful predictors of plant and microbial toxicity in soils and thus used total metal concentrations to develop normalisation relationships. In contrast, a number of other studies have reported various extractable measures to be better than total concentrations ( Posthuma & Notenboom 1996; Vijver et al. 2001; Nolan et al. 2005; Menzies et al. 2007).
McLaughlin et al. (2010) in a review of how to derive soil standards for trace elements concluded ‘it is difficult to conclude that one particular extractant or type of extractant is superior to others in predicting the trace element bioaccumulation and toxicity across a range of soils or organism endpoints’. There is also considerably more toxicity data expressed as total metal concentration. A further issue to be considered in development of EILs using extractable concentrations of contaminants would be the significant analytical challenge for many laboratories to consistently extract and accurately determine the low concentrations of contaminants found in partial extracts of soil.
One disadvantage of using total contaminant concentrations instead of a partial extract of soil designed to measure bioavailability is that different sources of contamination, having differing bioavailability, are not differentiated. However, for a screening level risk assessment such as the use of EILs, use of total concentrations is protective.
For the purposes of developing EILs (which are used across soils with a wide range of properties), there is some evidence from both overseas and Australia that, at least for metals, extractable concentrations in soil may not necessarily be better measures of bioavailability than total concentrations.
The use of normalisation relationships is an attempt to minimise the effect of soil characteristics on the toxicity data so the resulting toxicity data will more closely reflect the inherent sensitivity of the test species. If toxicity data more closely reflects species sensitivity, then a more accurate estimate of the soil concentration that should protect a certain percentage of species and soil processes can be derived. Normalisation relationships are also used to extrapolate ACL values determined for the Australian reference soil out to soils with a range of physicochemical properties (that is, different soils). To normalise toxicity data, empirical relationships are needed between soil characteristics and toxicity data. An example of a relationship between toxicity and a soil property is given in Figure 3, which shows how toxicity values increase with increasing soil pH.
Normalisation relationships are relatively simple empirical relationships between the toxicity or plant uptake data for a single contaminant to one species and the physicochemical properties of the soils where the tests were conducted. These empirical relationships are usually obtained using data from laboratory studies in which a single species is exposed to a single contaminant in different soils. Normalisation relationships have generally been developed using linear regression analysis techniques including forward and backward step-wise regression (Smolders et al. 2004; Rooney et al. 2006; Broos et al. 2007; Warne et al. 2008a) or partial least squares (PLS) regression (Lock & Janssen 2001). It is important that only soil physicochemical properties that are not significantly correlated to each other are used to develop normalisation equations. Although there are no generally accepted rules, researchers have generally only reported or recommended the use of normalisation equations that have coefficients of determination (r2) or adjusted coefficients of determination (adj r2) greater than 0.5 (that is, they explain more than 50% of the variation in toxicity values). This is quite reasonable as, if a relationship does not explain at least 50% of the variation, then using it to normalise other toxicity data could introduce considerable error.
A number of studies have successfully developed normalisation relationships for plants, microbial processes and soil invertebrates. The main soil characteristics affecting the toxicity of inorganic contaminants appear to be pH, clay content, cation exchange capacity and organic matter content (Lock & Janssen 2001; Smolders et al. 2003; Smolders et al. 2004; Rooney et al. 2006; Song et al. 2006; Broos et al. 2007; Warne et al. 2008a, 2008b).
![]()

Figure 3. An example of the effect that soil pH can have on toxicity values (shaded diamonds). Toxicity data shown are SIN EC 10 from the NBRP program.
Normalisation equations can, in principle, be developed for any combination of contaminant, species, and toxicity end point. However, they should only be developed using ecologically relevant species, measures and toxicity end points for the ecosystem that is being protected. In addition, it is preferable from an implementation point of view, that relatively easy and relatively cheap-to-measure, accurate, repeatable soil characteristics are used to derive normalisation relationships. Otherwise, the costs and difficulty of determining unusual soil characteristics will inhibit application of the relationships.
In Australia, empirical relationships have been obtained between soil characteristics and toxicity data for a limited set of contaminants and end points to date. Examples of relationships between toxicity and soil characteristics from the NBRP program are:
Microbial (substrate induced nitrification SIN) see also Figure 3
SIN log EC10 Zn = 0.55*pH – 0.55 R2 = 0.74 (equation 9)
Plant (toxicity)
log EC10 Zn = 0.271 * pH + 0.702 * log CEC adj. R2 = 0.66 (equation 10)
where pH is the soil pH (0.01 M CaCl2), CEC is the cation exchange capacity, EC10 is the concentration that causes a 10% effect.
Normalisation relationships are currently limited to a few combinations of contaminants, species and countries from which the soils are obtained. The lack of normalisation equations for a wide variety of species can be overcome by applying the relationships to species other than those for which they were derived (EU 2006b). However, this practice should only be conducted if it could be expected that the contaminant would exert its toxicity in the same manner as to the other species and the application of the normalisation relationship leads to a decrease in the range of toxicity values for the other species (EU 2006b).
The lack of normalisation equations for Australian soils can be overcome by using relationships developed with soils from other countries, particularly Europe and America. However, these normalisation relationships should only be used when they are derived from soils similar to Australian soils and/or if their validity for Australian soils has been assessed and found suitable[6]. The importance of this was shown by a study of Broos et al. (2007). This study assessed the normalisation relationships of Smolders et al. (2004) and Oorts et al. (2006) and re-analysed the data after removing microbial toxicity data for soils with OC concentrations greater than those found in Australian soils. This resulted in a change of soil characteristics, mainly explaining the variance in the toxicity data. For the initial data set, OC was the most important factor explaining the toxicity of Zn and Cu to nitrifying microorganisms but without the high OC soils, pH became the main explanatory soil property.
Normalisation relationships usually take the form of:
Toxicity data = a * soil property ± b (equation 11)
where a is the gradient of the regression and b is the y-intercept. The y-intercept is a measure of the inherent sensitivity of the test species used to derive the normalisation relationship—and each species will have a unique y-intercept. Thus, when applying normalisation relationships to other species, the toxicity data should only be transformed using the gradient (that is, a in equation 11) of the normalisation relationship (EU 2006).
A second option to overcome the lack of normalisation relationships in the literature is to examine the currently available toxicity data, and use regression analyses on the collated data to determine if a significant relationship exists between toxicity thresholds and soil characteristics.
Normalisation relationships from field studies are preferred over those from laboratory studies. All the normalisation relationships for toxicity apart from those developed by Broos et al. (2007) and Warne et al. (2008b) model laboratory-based data (Rooney et al. 2006; Smolders et al. 2003; Smolders et al. 2004; Oorts et al. 2006; EU 2006; Song et al. 2006; Warne et al. 2008a). Warne et al. (2008b) found that field-based normalisation relationships gave much more accurate estimates of field phytotoxicity than laboratory-based normalisation equations. Therefore, field-based normalisation relationships should be used to model field-based phytotoxicity data in preference to laboratory-based normalisation relationships. It is, however, realised that the current lack of the field-based normalisation relationships will unavoidably necessitate the use of laboratory-based relationships, despite their limitations.
If there are normalisation relationships for a toxicant, then the toxicity data should be normalised to a reference soil with a specified set of soil characteristics before the data is used in the SSD to derive the ACL value. Therefore, a reference soil for Australia should be used to normalise all the toxicity data (see Table 12). The specific setting of the Australian reference soil does not affect the EILs; however, all data should be normalised to the same chosen setting. Furthermore, it does not matter if all data is normalised to different settings and then an ACL value is calculated using the SSD method, or if one Australian setting is used, an ACL value is calculated and then the normalisation equation is used to calculate ACLs for different soil settings. This is because of the statistical methodology behind the SSD and normalisation approach.
Figure 4 shows how normalisation of toxicity data leads to a significant decrease in variation in toxicity values for a species (from the blue to the purple points in the figure). Therefore, the normalised toxicity data more accurately reflects the inherent sensitivity of each species.
![]()
Figure 4. Example of the effect of normalising using microbial toxicity data from the National Biosolids Research Program. The red arrows show how each toxicity value was normalised. The blue and pink arrows show the variation in toxicity values for the non-normalised and normalised data respectively. In this case the toxicity data was normalised to a pH of 6.
In general, there are three main methods to derive SQGs. These are in order of increasing complexity: the geometric mean method, AF methods and SSD methods. They are discussed below.
The SSD methods are statistical methods to calculate a soil concentration that protects a specified number of species and/or soil processes. Briefly, all SSD methods use toxicity data obtained from tests on individual species and fit a statistical distribution to the data to derive a concentration that should protect any selected percentage of species in the ecosystem being considered.
There are essentially four different SSD methods that have been used to derive EQGs:
The Stephan et al. (1985) method was the first SSD method developed. It is used by the USA to derive its WQGs (US EPA 1986) and was adopted by South Africa to derive freshwater guidelines (Roux et al. 1996).
Limitations of this method are that by using a log-triangular distribution it assumes there is a threshold toxicity value, below which no detrimental effects will occur, and the scientific literature and risk assessment theory does not support such an concept (Okkerman et al. 1991; OECD 1992; Emans et al. 1993; Pedersen et al. 1994; NZ Ministry of the Environment 1996) and it uses an arbitrary AF of two without any justification (Hart et al. 1995; NZ Ministry of the Environment 1996). As early as 1995, the US EPA recognised that the method required updating (Delos 1995). For the above reasons, this method was not considered for the derivation of the Australian and New Zealand WQGs (Warne 1998). At least partially due to the limitations of the Stephan et al. (1985) method, South Africa has adopted the more advanced Burr type III SSD method (Shao 2000) for its marine water quality guidelines (Warne et al. 2004a, 2004b).
In the late 1990s, the Aldenberg and Slob (1993) method was viewed as the preferred and most scientifically defensible SSD method. It was recommended over the Wagner and Løkke method by the OECD and subsequently adopted (OECD 1995). The Dutch used the Aldenberg and Slob method to derive their WQGs and SQGs. This reflected the research that the Dutch had undertaken to assess the scientific validity of this method (Emans et al. 1993; Okkerman et al. 1991, 1993). One drawback of the Aldenberg and Slob method compared to the Wagner and Løkke method was its use of the log-logistic distribution. There is no theoretical basis for the sensitivity of species to conform to a logistic distribution (Forbes & Forbes 1993). In fact, Aldenberg and Slob (1993) stated that the log-logistic distribution was chosen because it has ‘practical mathematical features that make the calculations of statistical confidence intervals relatively easy’. Aldenberg and Jaworska (2000) overcame the mathematical difficulties associated with using the normal distribution to develop a log-normal equivalent method to the Aldenberg and Slob method. The Aldenberg and Jaworska method has since been adopted by the Dutch to derive their WQGs and SQGs (Crommentuijn 2000a, 2000b). All of the above methods attempt to fit a single statistical distribution to the toxicity data.
The draft Australian and New Zealand WQGs (ANZECC & ARMCANZ 1999) adopted the Aldenberg and Slob SSD method. However, during the derivation of the TVs it was found that in more than 33% of cases where the Aldenberg and Slob method could be used, based on meeting the minimum data requirements of the method, the data did not have a log-logistic distribution. Therefore, strictly speaking, it was invalid to use the Aldenberg and Slob SSD method. This meant that for many contaminants an AF method had to be used. As there is no theoretical reason why species sensitivity must conform to a logistic distribution, there is no reason why other distributions cannot be considered. This issue was first realised by Shao (2000) and he therefore recommended that a family of distributions, the Burr type III (BT III) be used to fit to the toxicity data, rather than a single distribution as with the other SSD methods. Other authors (Maltby et al. 2003; Kwok et al. 2007) have since also adopted a more flexible approach to the statistical distributions being fitted to the data, whereby the distribution that best fits the data is used to derive the EQG or to determine the ecological risk.
The variety of shapes that BT III distributions can have is large (Shao 2000), including the log-logistic distribution and approximations of the log-normal and log-triangular distributions. Thus, attempting to fit a BT III distribution to any given toxicity data set has a greater probability of success than attempting to fit only the log-logistic distribution.
This method is guaranteed to fit a statistical distribution to the toxicity data at least as well as the Aldenberg and Slob method because the log-logistic distribution is a BT III distribution (Shao 2000). Greater detail about the BT III method is provided in Shao (2000).
The main difference between the various SSD methods is the statistical distribution that they fit to the data. For that reason, the following explanation of how SSDs work is generic.
In SSD methods, each species is given equal weighting and a single value is used to represent the sensitivity of each species. However, there is usually multiple toxicity data for each species which requires some manipulation. The rules governing this manipulation were presented earlier in this Schedule. The data is then entered into SSD software such as ETx (Aldenberg & Jaworska 2000) or BurrliOZ (Campbell et al. 2000). The SSD calculates the cumulative frequency of the species sensitivity data by ranking the data from lowest to highest and then using the formula:
cumulative frequency = rank * [100/(n + 1)] (equation 12)
The cumulative frequency for each species is then plotted against the concentration that represents the sensitivity of each species. A typical SSD plot is shown in Figure 5 below. In the case of the Stephan et al. (1985), Wagner and Løkke (1991), Aldenberg and Slob (1993), and Aldenberg and Jaworska (2000) methods that fit one specific distribution to the toxicity data, statistical tests (for example, the Kolmorogorov Smirnov test or the Anderson-Darling test) are used to determine if the toxicity data fits the selected distribution. The more flexible SSD methods, for example, BT III and the approach adopted by Maltby et al. (2003) and Kwok et al. (2007), use statistical methods (for example, maximum likelihood methods, Anderson-Darling test) to determine which particular statistical distribution best fits the toxicity data. In doing this, the SSD methods estimate the parameters that mathematically describe the selected distribution. Because the equation that describes the selected distribution is known, it is very simple to calculate the concentration that should theoretically protect any chosen percentage of species or permit any chosen percentage of species to experience toxic effects. To do this, the cumulative frequency that corresponds to the percentage of species to be protected is entered into the equation for the distribution that best fitted the toxicity data. Thus, the 5th percentile of the selected distribution becomes the concentration that, if not exceeded, will protect 95% of species and the 10th percentile will protect 90% of species, and so on. The resulting concentrations are generally referred to in Europe as hazardous concentration (HC) values, while in Australia and NZ, Hong Kong and South Africa they are termed protective concentration (PC) values. The number following HC or PC indicates the percentage of species that should be harmed or should be protected respectively. More detailed information on each SSD method can be obtained from the original documents cited above and in the thorough review of SSD methods by Posthuma et al. (2002).
Figure 5. A typical SSD plot. The example provided is output from the BurrliOZ program using EC10 values for plants (field data NBRP)
The toxicity data used to derive a PC value is only a sample of the total species in the ecosystem being protected. As with any sampling program, different distributions could be obtained depending on the species that form the sample. Therefore, different samples could lead to different PC values for the same contaminant being calculated. Aldenberg and Slob (1993) overcame this problem by developing two confidence limits: 95% and 50% for the HC or PC values respectively. These confidence limits indicate the degree of certainty that the calculated HC value will protect the selected percentage of species. Thus, a HC5 95 value means that there is a 95% certainty that the concentration will protect at least 95% of species in an ecosystem. The Dutch used the HC5 95 values as their long-term aspirational goal for water quality. In the Australian and New Zealand WQGs (ANZECC & ARMCANZ 2000) and in jurisdictions that have adopted their methodologies (that is, Hong Kong and South Africa in its marine water quality guidelines) confidence intervals are not used. This was developed because the 95% confidence limits were not deemed to be statistically robust (Fox 1999). Additionally, if the sample size is large, the 50th percentile will approximate the median of estimates of the PC value. Thus, the 50th percentile should equal the HC5 50.
All SSD methods make a series of assumptions. In the early 1990s, the SSD methods received considerable criticism from Calabrese and Baldwin (1993), Forbes and Forbes (1993), Schudoma (1994), and Smith and Cairns (1993), and some doubted whether the SSD methods were in fact better than the AF methods.
The key criticisms were:
A number of the other assumptions made by SSD methods were also attacked by these authors; however, these were assumptions made by all methods of deriving EQGs. There is considerable experimental support for the SSD methods (Emans et al. 1993; Okkerman et al. 1991, 1993). In addition, organisations such as the OECD compared both the SSD methods and AF methods and concluded by recommending the SSD methods (OECD 1995). An overview of the criticisms and support for the SSDs is provided in Warne (1996) and a more condensed version in Warne (1998). Several authors including Forbes and Calow (2002a) have now changed their position considerably and support SSDs while acknowledging their limitations. SSD methods are now well established and widely used in deriving EQGs and conducting ERAs. For example, SSD methods are the preferred method of deriving the EU soil and water quality guidelines (ECB 2003; EU 2006b).
A potential weakness of SSD methods, and indeed of all modelling methods, is that as the quantity of data used decreases the effect of the sample used increases dramatically. Initial studies by the Danish EPA (Pedersen et al. 1994) and the OECD (1995) indicated that WQGs derived using data sets containing less than five values were very dependent on the spread of the values, whereas for data sets containing five or more values this effect was markedly reduced. Subsequent more rigorous work by Newman et al. (2000), Forbes and Calow (2002b) and Wheeler et al. (2002) indicated that toxicity data for between 10 and 30 species was necessary for the resulting limit values to be stable irrespective of the sample. To calculate an HC5/PC95 value using empirical methods, at least 20 species are needed, and 100 species are needed for an HC1/PC99 value (Forbes & Calow 2002b). Using non-parametric methods, Newman et al. (2000) estimated for 30 toxicants that between 15 and 55 (median of 30) species per toxicant were needed, while Wheeler et al. (2002) estimated a minimum of 10 to 15 species per toxicant were needed. The decision by the regulating agency about the appropriate number of species is often arbitrary (Pennington 2003): US EPA requires at least eight species (US EPA 1999), the Dutch suggest ten (van Vlaardingen & Verbruggen 2007), the OECD between five and eight (OECD 1992, 1994) and Australia and New Zealand—five species (Warne 2001). It is worth remembering that the above estimates are based on available SSDs that tend to include data from only a small fraction of taxonomic and other groups present in nature. If data were available for a larger range of organisms, the number of species for which data is required may increase. If this occurred, then the findings of Newman et al. (2000), Wheeler et al. (2002), and others would have underestimated the number of species required for estimating the SSDs. Reflecting these findings, the EU has required that future WQGs need toxicity data for at least ten species that belong to at least eight taxonomic groups and an additional assessment factor of 15 to the PC95 should be considered (ECB 2003).
SSD methods have a number of strengths:
The limitations of the methods include:
In AF methods, all available toxicity data for a contaminant is collated. Then the lowest toxicity value is divided by a constant that is variously called an assessment factor, uncertainty, application or safety factor. Typically the AFs are 10, 100 or 1000. The magnitude of the AF used to derive an EQG is inversely related to the perceived environmental relevance of the toxicity data; that is, the more environmentally realistic the toxicity data, the smaller the AF and vice-versa. This approach for deriving EQGs was first proposed by Hart et al. (1995) and was adopted from methods used in human health to derive average daily intakes (Cotruvo 1988; Calabrese & Baldwin 1993). The AF method is used to derive both soil and water quality guidelines in numerous countries.
Depending on the toxicity data available, up to three extrapolations can be made by AF methods, with each extrapolation typically given an AF of 10. The extrapolations are laboratory-to-field, acute-to-chronic, and interspecies, and are designed to compensate for inadequacies in the available toxicity data. The magnitude of the various AFs and the type and magnitude of the extrapolations that are inherently assumed by the AFs used in the modified US EPA (OECD 1992) and CCME (1991) methods are presented in Table 18 below.
The field-to-laboratory extrapolation accounts for the supposition that laboratory studies tend to underestimate the toxicity in the field. Proposed reasons for this include: laboratory tests being conducted on animals that are robust and easily bred/maintained in the laboratory rather than ‘sensitive’ species, life stages that are not tested in the laboratory may be more sensitive to toxicants (Hart 1996), and all the limitations associated with single species toxicity tests that are discussed in Warne (1998).
It is also possible for laboratory-based experiments to overestimate the toxicity in field situations. This can arise if laboratory experiments use freshly spiked soils with minimal ageing period, which overestimates the bioavailability compared to field bioavailability.
The acute-to-chronic extrapolation is extensively used to derive WQGs because the vast majority of toxicity data is acute whereas chronic data is preferred for environmental protection. The CCME method (CCME 1991), like the original US EPA method (US EPA 1986), uses an ACR derived from another species for the same contaminant in preference to a generic ACR. When a contaminant-specific ACR is not available, then CCME (1991) and the US EPA (1986) use a generic ACR. CCME (1991) uses an ACR of 2 or 10 depending on the environmental persistence of the contaminant, while the modified (OECD 1995) and unmodified US EPA (1986) methods use one generic ACR of 10.
However, an acute-to-chronic extrapolation is not used in soil guideline value derivation. An acute-to-chronic extrapolation should only be used for short-term contact exposure studies. Such tests are a very short-term acute toxicity test performed on direct dermal contact using earthworms, which might not represent exposure in soils accurately. The test will very likely give toxicity values that are an underestimation of chronic exposure toxicity data.
Most AF methods used in most jurisdictions have minimum data requirements. When these are not met then an interspecies extrapolation is used. This is used because there is increased uncertainty in deriving guideline values from such a small sample size.
Table 18. The assessment factors, types and magnitudes of the extrapolations used in the modified US EPA and CCME methods
Available toxicity data | Type of extrapolation | Modified US EPA methoda | CCME methodb |
Chronic NOEC (for the US EPA) | Field-to-laboratory | 10 | 10 |
Acute LC50 or EC50 | Field-to-laboratory and acute-to-chronic | 100 | ACR or |
Acute LC50 or EC50 for one | Field-to-laboratory and acute-to-chronic and interspecies | 1000 | ACR or |
a It is assumed toxicity data is available for at least an algae, a crustacean and a fish (OECD 1992).
b Assumes that toxicity data is available for at least three species of fish, of which two must be chronic; two invertebrates, one of which should be planktonic; and a freshwater vascular plant or algae (CCME 1991).
c An AF of 50 is used for non-persistent contaminants while 100 is used for persistent contaminants when no ACR is available.
d Where data is not sufficient to meet the requirements set in b, then interim WQGs are derived (CCME, 1991).
Criticisms of the AF approach revolve around the scientific validity of AFs, the magnitude of the AFs, and whether or not the method is consistent with a risk framework and the principle of ecologically sustainable development. Many scientists and organisations have acknowledged the arbitrary nature of AFs, that they have no theoretical scientific basis and are purely empirical (Hart 1974; Nicholson 1984; Kooijmand 1987; Okkerman et al. 1991; OECD 1992; Schudoma 1994; Rand et al. 1995; OECD 1995; Warne 1998). Goldberg (1975) asserted that using AFs was tantamount to admitting that information essential for risk assessments was lacking.
Nicholson (1984) considered that:
’There is little scientific basis for application factors except that they are the result of careful judgement there is little evidence, in most cases, that the arbitrary value chosen is indeed the best choice, i.e. whether a particular value for an application factor will provide ‘adequate’ protection and whether a less (or more) stringent value would be more appropriate.’
The fact that there is no universally accepted magnitude for AFs (as seen in Table 18) confirms their arbitrary nature. The AF method ignores all other data except the lowest and is therefore an example of the ‘worst-case scenario’ type of approach. Such a procedure is at odds with a risk-based approach, which requires an array of data in order to derive estimates of the probability of certain toxicological events occurring. Risk-based concepts and procedures are central to many of the more recently adopted scientific, social and political paradigms within Australia including the current Australian and New Zealand Guidelines for fresh and marine water quality (ANZECC & ARMCANZ 2000).
There has been considerable discussion in the scientific literature about the appropriate size of AFs. There are numerous examples of where AFs should be less than 10 and equally numerous examples of where they should be considerably larger (refer to Warne (1998) and Chapman et al. (1998) for detail). Chapman et al. (1998) concluded that the discussion about the size of the AFs is ’to some extent futile … because no one set of factors has universal applicability’. Ultimately, AFs are a measure to address a lack of knowledge and as soon as that knowledge is available, AFs should no longer be used.
The strengths of AF methods are that:
The weaknesses of AF methods are that:
Reflecting the above limitations, many countries only use AF methods to derive SQGs and/or WQGs when SSD methods cannot be used. For example, the Australian and New Zealand (ANZECC & ARMCANZ 2000), OECD (1995), the Netherlands (Crommentuijn 2000), Canadian (CCME 2006), Danish (Bro-Rasmussen et al. 1994) and South African (Roux et al. 1996) guidelines all now use a statistical extrapolation method in preference to an AF method, which is only used when there is insufficient data.
The US EPA has developed ecological soil screening levels (Eco-SSLs) for sites where terrestrial organisms may be exposed directly or indirectly to contaminated soil, using the geometric mean method. The geometric mean[7] method uses all the toxicity values at the highest relative bioavailability score for which sufficient data existed (that is, ≥3 data points). Thus, the Eco-SSL is really the geometric mean of the sensitivities of all organisms for which there is toxicity data in the most bioavailable situation. By using the geometric mean approach, there is no consistent level of protection being provided (that is, different percentages of species will be protected). This is not a particularly conservative approach for the soil ecosystems where the contaminant is most bioavailable. However, the percentage of species that could experience toxic effects will be less and the degree of conservatism greater in the soils where the contaminant is less bioavailable.
Geometric means are also used in the manipulation of toxicity data prior to use within SSD methods. However, the manner in which the geometric means are implemented is quite different to that of the US EPA Eco-SSLs. The geometric mean approach is a combination of the worst-case scenario and risk-based approaches. It is a worst-case scenario as it derives Eco-SSLs for the soil in which the contaminant is most bioavailable. It is consistent with risk-based approaches as it does not attempt to protect all species.
The strengths of the geometric mean method are that:
The limitations of the method are that:
In deciding which of the above methods would be best to derive EILs, it is important to recognise the role of EILs. They are a concentration above which further investigation should be conducted. Therefore, if the contaminant concentration does not exceed the EIL, then it is assumed that the situation does not warrant further investigation and is, in fact, safe. Therefore, EILs need to be reasonably conservative. Other considerations are scientific validity, ease of use and interpretation and consistency with existing Australian environmental management systems.
Secondary poisoning can occur if a contaminant biomagnifies, that is, it accumulates in organisms’ tissue and the concentration increases with each trophic level in a food web (for example, soil—earthworms—birds—predatory birds). The species most at risk are those in the higher trophic levels in a food web, i.e. the predators. Examples of contaminants that biomagnify and have deleterious effects on predators include DDT, Cd and PCBs (Morrissey et al. 2005; Jongbloed et al. 1996).
The vast majority of environmental toxicity data is on direct exposure to contaminants from the ambient environment (that is, soil, water or air) and not from food. Therefore, if contaminants are biomagnified, then normal toxicity data and EILs based on such data may underestimate the impact the contaminant has on the environment and communities. To overcome this problem, contaminants that biomagnify need to be identified and biomagnification needs to be considered in deriving the EIL for those contaminants.
Secondary poisoning is taken into account in the soil quality guidelines of several countries, including Canada (CCME 2006), USA (US EPA 1996) and the Netherlands (Van de Plassche 1994). However, not all countries consider secondary poisoning in their SQGs, for example, Germany (BBodSchV 1999).
There are three methods for deriving EILs that account for biomagnification:
These methods are critically assessed below.
There are three slightly different biomagnification algorithms. The main difference between them is whether ingestion of soil is considered (the US EPA and Canadian methods) or not (the Dutch method).
The US EPA methodology (US EPA 1996), which accounts for soil ingestion, calculates the secondary poisoning SQG (SQGsp) by:
![]() (equation 14)
(equation 14)
where SQGsp is the soil quality guideline that accounts for secondary poisoning and is expressed in mg/kg, the toxicity reference value is expressed in mg contaminant/kg prey tissue, FIR is food intake rate (kg food [dry weight]/ kg body weight [wet weight] /day), Ps is the proportion of the diet that is soil (%) and BAFij is the bioaccumulation factor for contaminant ‘i’ by species ‘j’ (unitless).
The Canadian methodology (CCME 2006) is based on daily intake models similar to derivation of maximum human daily uptake models. The Canadian methodology takes into account direct soil ingestion and bioaccumulation through the food chain.
SQGs are thereby calculated using the following equation:
![]() (equation 15)
(equation 15)
where SQG2C refers to the soil quality guideline for soil and food ingestion for the secondary consumer (mg/kg dry weight soil), DTED2C is the daily threshold effects dose for the secondary consumer (mg/kg body weight-day), BW2C is the body weight of the species used in the DTED2C (kg), SIR2C is the soil ingestion rate for the species used in the DTED2C (kg dry weight soil/day), BF is the bioavailability factor (unitless), FIR2C is the food ingestion rate for the species used in the DTED2C (kg dw food/day) and BAF2 is the bioaccumulation factor (unitless) (CCME 2006).
The Dutch methodology developed by Van der Plassche (1994) or Romijn et al. (1991) does not account for soil ingestion and calculates the SQG by:
![]() (equation 16)
(equation 16)
where SQGsp is the soil quality guideline that accounts for secondary poisoning expressed in mg/kg, NOEC predator is the NOEC for a predator expressed as mg contaminant/kg prey tissue, BCFprey is the bioconcentration factor of the contaminant for a prey species expressed as a ratio of concentration in the prey and in the soil. If the BCFprey is unknown, the BCF was predicted based on the log Kow of the contaminant using QSARs.
The above methods were not adopted in the Australian and NZ WQGs because of ‘the lack of relevant data’ and as there is ‘no formal and specific guidance on how to take information on bioaccumulation into account when deriving water quality guidelines’ (ANZECC & ARMCANZ 2000). Food web approaches were not advocated because they are ‘very complex and require extensive data sets, which are not available for the majority of contaminants’ (ANZECC & ARMCANZ 2000). These data sets include toxicity data for top predators, biomagnification and bioaccumulation data and dietary information for the species. For terrestrial ecosystems, Australian data needed for a food web modelling approach is even scarcer. The paucity of Australian data was the main reason why a proposed food web methodology for deriving EILs was not incorporated into this guideline.
However, biomagnification algorithms are currently the best available methodology to set EILs that protect top predators if the necessary data sets are available.
The biomagnification default factor method refers to dividing the normal SGQ by a biomagnification factor to protect the higher predators. Predators are assumed to have the same sensitivity to the contaminant as other species, but as biomagnification occurs in the food web, the SQG is divided by a default biomagnification factor to protect the predators. This default biomagnification factor could be derived by collating biomagnification values for similar contaminants and then a specific percentile value on a log-normal basis could be adopted as the default BMF. If biomagnification values are not known, a conservative default biomagnification factor could be set (for example, 10). This is a simple and easily understood method but it could under-protect for some combinations of species and contaminants and over-protect for others. This methodology can also result in very conservative limit values.
Increasing the percentage of species to be protected is an indirect way of addressing biomagnification and was used in the Australian and New Zealand WQGs (ANZECC & ARMCANZ 2000). For example, the level of protection was raised from 95% to 99% for slightly to moderately modified ecosystems. It is a simple method but not necessarily scientifically rigorous. As it does not directly address biomagnification, it cannot be guaranteed that the resulting limit values will provide sufficient protection. Furthermore, this methodology might give very conservative limit values which in some cases could be lower than background concentrations. This occurred when PC99 values were derived for some metals (Warne pers. comm.).
Metals and metalloids are naturally present in soils. Natural (background) concentrations of metals in soils depend on the parent rock from which the soil originated and are highly variable. Some authors (Reimann & Garrett 2005) argue that natural background concentrations no longer exist anywhere in the world due to man-made activities and global transport of contaminants. Therefore, the term ambient background concentration (ABC) as suggested by Zhao et al. (2007) is used rather than background concentration.
Metal concentrations in soils are easily and quickly measured; therefore, the preference is to directly measure the ABC in known unpolluted reference soils. However, finding a similar unpolluted reference soil to the contaminated soil is not always possible for a wide variety of reasons. The complexity and problems associated with measuring the ABC are discussed in a series of papers in Human and Ecological Risk Assessment, vol. 9 (2003) and by Reimann and Garrett (2005). Reliable ABC values for a soil with similar physicochemical and structural properties to the soil being investigated cannot always be obtained or the measured values are compromised in one or more ways. If reliable background concentrations cannot be obtained, then a modelling method should be used.
A model able to predict the background concentrations of metals in Australian soils was developed by Hamon et al. (2004). In this study, a large number of remote sites in Australia and South-East Asia were surveyed for metal concentrations in soil. Principal component analysis revealed strong associations of many metals (for example, As, Co, Cr, Cu, Ni, Pb) with structural elements of soil minerals (Fe and Mn). Linear regressions were developed that permit the prediction of background soil metal concentrations using only Fe or Mn concentrations (Figure 6).
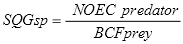
Figure 6. Example relationships between the logarithm of iron concentration of soil and background Cu and Ni concentrations (modified from Hamon et al. 2004). The red and black lines are the 95th and 50th percentile of the relationships respectively.
The equations developed by Hamon et al. (2004 [Table 15, Section 2.4.9.1]) can be used to estimate the background concentration. Hamon et al. (2004) calculated the ‘background concentrations’ using the equation that encompassed the upper 95th percentile of the data. However, Zhao et al (2007) argued that this approach is not conservative as the poorer the relationships, the larger the 95th percentile will be and hence the larger the estimates of ABC will be. They argue that this may lead to under-protection of soils (by deriving larger ABCs which are added to limit values base on added metal concentrations). Given the above and the purpose of EILs, the 50th percentile of the data (that is, the regression equation) should be used to estimate ABC values.
The relationships developed by Hamon et al. (2004) take the form
ABC = a* log Fe or Mn + b (equation 17)
To calculate the ABC, measure the Fe and Mn concentration in the soil (expressed in %) using aqua regia digestion (Hamon et al. 2004), and substitute the appropriate metal concentration into the appropriate equation. It is, however, necessary to ascertain that the Fe and Mn content of the soil at the site in question is not elevated by contamination. These elements are normally determined in chemical analysis of soils to determine total metal concentrations and therefore minimal extra cost is involved.
Most organic contaminants of interest to contaminated sites are xenobiotics, hence they have no natural background concentration. Notable exceptions to this include lipids and fats, hormones (for example, oestrogen, testosterone), fatty acids, alcohols, hydrocarbons, polycyclic aromatic hydrocarbons and dioxins. Therefore, ABCs will have to be generated by direct measurement or a default ABC of zero (Crommentuijn et al. 2000b) could be assumed. There are no equivalent models to that of Hamon et al. (2004) available for organic contaminants.
For pyrogenic and naturally occurring organic contamination, a site-specific assessment should be conducted to determine if the measured concentrations are background concentrations for that region. If a site-specific assessment is conducted, then the upper 80th percentile of the ABCs should be used as the background as per the Australian and New Zealand WQGs (ANZECC & ARMCANZ 2000). However, even if they are considered ABCs, this does not imply that there is no risk to terrestrial biota.
Aldenberg, T & Jaworksa, JS 2000, ‘Uncertainty of the hazardous concentrations and fraction affected for normal species sensitivity distributions’, Ecotoxicology and Environmental Safety, vol. 46, pp. 118.
Aldenberg, T & Slob, W 1993, ‘Confidence limits for hazardous concentrations based on logistically distributed NOEC toxicity data’, Ecotoxicology and Environmental Safety, vol. 25, pp. 4863.
Alloway, BJ 1995, Heavy metals in soils, 2nd edn, Blackie Academic and Professional, London, UK.
ANZECC & ARMCANZ 1999, National water quality management strategy, Draft Australian and New Zealand guidelines for fresh and marine water quality. Australian and New Zealand Environment and Conservation Council & Agriculture and Resource Management Council of Australia and New Zealand, Canberra, Australia.
ANZECC & ARMCANZ 2000, National water quality management strategy. Australian and New Zealand guidelines for fresh and marine water quality, Australian and New Zealand Conservation Council & Agriculture, and Resource Management Council of Australia and New Zealand, Canberra, Australia.
Bääth, E, Diaz-Ravina, M & Frostegard, A 1998, ‘Effect of metal-rich sludge amendments on the soil microbial community’, Applied and Environmental Microbiology, vol. 64, pp. 238245.
Barrow, NJ 1986, ‘Testing a mechanistic model, IV, Describing the effects of pH on zinc retention by soils’, Journal of Soil Science, vol. 37, pp. 295302.
Basta, NT, Ryan, JA & Chaney, RL 2005, ‘Trace element chemistry in residual-treated soil: key concepts and metal bioavailability’, Journal of Environmental Quality, vol. 34, pp. 4963.
BBodSchV 1999, Bundes-Bodenschutz und Altlastenverordnung (BBodSchV), vom 12. Juli 1999, (Federal soil protection and contaminated sites ordinance, dated 12 July 1999), Bundesgesetzblatt I, 1554.
Briggs, GG 1981, ‘Theoretical and experimental relationships between soil adsorption, octanolwater partition coefficients, water solubilities, bioconcentration factors, and the parachor’, Journal of Agricultural and Food Chemistry, vol. 29, pp. 10501059.
Brix, KV, DeForest, DK & Adams, WJ 2001, ‘Assessing acute and chronic copper risks to freshwater aquatic life using species sensitivity distributions for different taxonomic groups’, Environmental Toxicology and Chemistry vol. 20, no. 8, pp. 18461856.
Bro-Rasmussen, F, Calow, P, Canton, JH, Chambers, PL, Silva Fernandes, A, Hoffmann, L, Jouany et al. 1994, ‘EEC water quality objectives for chemicals dangerous to aquatic environments (List 1)’, Reviews of Environmental Contaminants and Toxicology, vol. 137, pp. 83110.
Broos, K, Warne, MStJ, Heemsbergen, DA, Stevens, D, Barnes, MB, Correll, RL et al. 2007, ‘Soil factors controlling the toxicity of Cu and Zn to microbial processes in Australian soils’, Environmental Toxicology and Chemistry vol. 26, pp. 583590.
Calabrese, EJ & Baldwin, LA 1993, ’Chemical-specific ecosystem MATC’, Performing ecological risk assessments, Lewis Publishers, pp. 165183, Boca Raton, USA.
Campbell, E, Palmer, MJ, Shao, Q, Warne, MStJ & Wilson, D 2000, ‘BurrliOZ: A computer program for the estimation of the trigger values for the ANZECC and ARMCANZ water quality guidelines’, in National water quality management strategy. Australian and New Zealand guidelines for fresh and marine water quality, Australian and New Zealand Conservation Council and Agriculture, & Resource Management Council of Australia and New Zealand, Canberra, Australia.
Carlon, C 2007 (ed.), Derivation methods of soil screening values in Europe. A review and evaluation of national procedures towards harmonization. European Commission, Joint Research Centre, ISPRA, EUR 22805-EN.
CCME 1991, ‘Appendix IX’, Canadian water quality guidelines, pp. IX-1IX-8, Canadian Council of Ministers for the Environment, Inland Water Directorate, Environment Canada, Ottawa, Canada.
CCME 1999, Canadian environmental quality guidelines, available online at: http://www.ccme.ca/publications/can_guidelines.html.
CCME 2006, A protocol for the derivation of environmental and human health soil quality guidelines, available online at: http://www.ccme.ca/assets/pdf/sg_protocol_1332_e.pdf.
Chapman, PM, Cardwell, RS & Chapman, PF 1996, ‘A warning: NOECs are inappropriate for regulatory use’, Environmental Toxicology and Chemistry, vol. 15, pp. 7779.
Chapman, PM, Fairbrother, A & Brown, D 1998, ‘A critical evaluation of safety uncertainty factors for ecological risk assessment’, Environmental Toxicology and Chemistry, vol. 17, pp. 99108.
Chapman, PM, McDonald, BG, Kickham, PE & McKinnon, S 2006, ‘Global geographic differences in marine metals toxicity’, Marine Pollution Bulletin, vol. 52, no. 9, pp. 10811084.
Connell, DW 1989, ‘Bioconcentration of lipophilic and hydrophobic contaminants by aquatic organisms’, in Connell, DW (ed.), Bioaccumulation of xenobiotic contaminants, CRC Press, Boca Raton, Florida, USA.
Cotruvo, JA 1988, ‘Risk assessment of carcinogenic and noncarcinogenic chemicals’, Critical Reviews in Toxicology, vol. 20, pp. 341367.
Crommentuijn, T, Polder, M & van de Plassche, E 1997, Maximum permissible concentrations and negligible concentrations for metals taking background concentrations into account, RIVM report number 601501 001, National Institute of Public Health and the Environment, Bilthoven, Netherlands.
Crommentuijn, T, Sijm, D, de Bruijn, J, van den Hoop, M, van Leeuwen, K & van de Plassche, E 2000a, ‘Maximum permissible and negligible concentrations for metals and metalloids in the Netherlands, taking into account background concentrations’, Journal of Environmental Management, vol. 60, pp. 121143.
Crommentuijn, T, Polder, M, Sijm, D, de Bruijn, J, van den Hoop, M & van de Plassche, E 2000b, ‘Evaluation of the Dutch environmental risk limits for metals by application of the added risk approach’, Environmental Toxicology and Chemistry, vol. 19, pp. 16921701.
Dean, JR & Scott, WC 2004, ‘Recent developments in assessing the bioavailability of persistent organic pollutants in the environment’, Trac-Trends in Analytical Chemistry, vol. 23, pp. 609618.
Delos, CG 1995, ‘Possible revisions to EPA’s procedure for deriving aquatic life criteria‘, Proceedings of the Water Environment Federation, 67th Conference and Exposition, vol. 4, Chicago, Illinois, USA, pp. 661667.
Diaz-Ravina, M & Bääth E 1996, ‘Development of metal tolerance in soil bacterial communities exposed to experimentally increased metal levels’, Applied and Environmental Microbiology, vol 62, pp. 29702977.
Dyer, SD, Belanger, SE & Carr, GJ 1997, An initial evaluation of the use of Euro/North American fish species for tropical effects assessments’, Chemosphere, vol. 35, no. 11, pp. 27672781.
Emans, HJB, van de Plassche, EJ, Canton, JH, Okkerman, PC & Sparenburgs, PM 1993, ‘Validation of some extrapolation methods for effects assessment‘, Environmental Toxicology and Chemistry, vol. 12, pp. 21392154.
EC 2008, ‘Environmental effects’, European Union voluntary risk assessment report: copper, copper II sulphate pentahydrate, copper(I)oxide, copper(II)oxide, copper chloride trihydroxide, European Commission, Brussels, Belgium, available online at http://echa.europa.eu/chem_data/transit_measures/vrar_en.asp.
ECB 2003, Technical guidance document on risk assessment, EUR 20418 EN/1, European Chemicals Bureau, Office for Official Publications of the European Community, Luxembourg.
ECETOC 1993, Aquatic toxicity database evaluation, ECETOC Technical report no. 56, European Centre for Toxicology of Chemicals, Brussels, Belgium.
EU 2006a, ‘Directive 2000/60/EC of the European Parliament and of the Council establishing a framework for community action in the field of water policy’, Official Journal of the European Communities, L327/1-72, European Union, Brussels, Belgium.
EU 2006b, ‘Terrestrial effects assessment’, Draft risk assessment report for nickel and nickel compounds, Section 3.1, Draft of May 11, 2006, European Union, Brussels, Belgium.
Fait, G, Broos, K, Zrna, S, Lombi, E & Hamon, R 2006, ‘Tolerance of nitrifying bacteria to copper and nickel’, Environmental Toxicology and Chemistry, vol 25, pp. 20002005.
Forbes, TL & Forbes, VE 1993, ‘A critique of the use of distribution-based extrapolation models in ecotoxicology’, Functional Ecology, vol. 7, pp. 249254.
Forbes, VE, Calow, P 2002a, ‘Species sensitivity distributions revisited: a critical appraisal’, Human and Ecological Risk Assessment, vol 8, pp. 473492.
Forbes, VE, Calow, P 2002b, ‘Extrapolation in ecological risk assessment: balancing pragmatism and precaution in chemical controls legislation‘, BioScience, vol. 5, pp. 249257.
Fox, DR 1999, ‘Setting water quality guidelines: a statistician’s perspective’, SETAC News, May, pp. 1718.
Fox, DR 2008, ‘Opinion. NECs, NOECs and ECx’, Australasian Journal of Ecotoxicology, vol. 14, no. 1, pp. 610.
Goldberg, L 1975, ‘Safety evaluation concepts’, Journal of the Association of Official Analytical Chemists, vol. 58, pp. 635644.
Gupta, SK, Vollmer, MK & Krebs, R 1996, ‘The importance of mobile, mobilisable and pseudo total heavy metal fractions in soil for three-level risk assessment and risk management’, Science of the Total Environment, vol. 178, pp. 1120.
Hamon, RE, McLaughlin, MJ, Gilkes, RJ, Rate, AW, Zarcinas, B, Robertson et al. 2004, ‘Geochemical indices allow estimation of heavy metal background concentrations in soils‘, Global Biogeochemical Cycles, vol. 18, GB1014, doi:10.1029/2003GB002063.
Hamon, RE, McLaughlin, MJ & Lombi, E 2007 (eds), Natural attenuation of trace element availability in soils, SETAC Press, Pensacola/Taylor and Francis, Boca Raton, Florida, USA.
Hart, BT 1974, A Compilation of Australian Water Quality Criteria. Australia Water Resources Council, Technical Paper 7, Australian Government Publishing Service, Canberra, Australia.
Hart, BT, Jones, MJ & Chapman, JC 1995, A process for the development of guidelines for the protection of aquatic life in New Zealand, Report to the New Zealand Ministry for the Environment, Water Studies Centre, Monash University, Melbourne, Australia.
Hart, BT 1996, A methodology for deriving aquatic guideline values for toxic contaminants, first draft, Report to the New Zealand Ministry for the Environment, Water Studies Centre, Monash University, Melbourne, Australia.
Heemsbergen, DA, Warne, MStJ, Broos, K, Bell, M, Nash, D, McLaughlin, MJ et al. 2009, ‘Application of phytotoxicity data to a new Australian soil quality guideline framework for biosolids‘, Science of the Total Environment, vol. 407, pp. 25462556.
Hobbs, DA, Warne, MStJ & Markich, SJ, 2004, ‘Utility of northern hemisphere metal toxicity data in Australasia‘, Setac Globe, vol. 5, no. 2, pp. 3839.
Hobbs, DA, Warne, MStJ & Markich, SJ, 2005, ‘Evaluation of criteria used to assess the quality of aquatic toxicity data‘, Integrated Environmental Assessment and Management, vol. 1, pp. 174180.
Hobbs, DA 2006, ‘Comparison of the sensitivity of Australasian and non-Australasian aquatic organisms to selected metals‘, MSc thesis, University of Technology Sydney, Australia.
Hoekstra, JA & van Ewijk, PH 1993, ‘Alternatives for the no-observed effect level‘, Environmental Toxicology and Chemistry, vol. 12, pp. 187194.
Hose, GC & van den Brink, PJ 2004, ‘Confirming the species-sensitivity distribution concept for endosulfan using laboratory, mesocosm, and field data’, Archives of Environmental Contamination and Toxicology, vol. 47, no. 4, pp. 511520.
Hulzebos, EM, Adema, DMM, Dirven-van Breemen, EM, Henzen, L & van Gestel, CAM 1991, ‘QSARs in phytotoxicity’, Science of the Total Environment vol. 109110, pp. 493497.
Jongbloed, RH, Traas, TP & Luttik, R 1996, ‘A probabilistic model for deriving soil quality criteria based on secondary poisoning of top predators’, Ecotoxicology and Environmental Safety, vol. 34, pp. 279306.
Jury, WA, Farmer, WJ & Spencer, WF 1983, ‘Use of models for assessing relative volatility, mobility, and persistence of pesticides and other trace organics in soil systems‘, in Saxena, J (ed.), Hazard assessment of chemicals, vol. 2, Academic Press, New York, pp 143.
Jury, WA, Farmer, WJ & Spencer, WF 1984, ‘Behavior assessment model for trace organics in soil: II, chemical classification and parameter sensitivity’, Journal of Environmental Quality, vol. 13, pp. 567587.
Kooijmand, SALM 1987, ‘A safety factor for LC50 values allowing for differences in sensitivity among species’, Water Research, vol. 21, pp. 269276.
Kwok, KWH, Leung, KMY, Chu, VKH, Lam, PKS, Morritt, D, Maltby, L et al. 2007, ‘Comparison of tropical and temperate freshwater species sensitivities to chemicals: implications for deriving safe extrapolation factors‘, Integrated Environmental Assessment and Management, vol. 3, pp.4967.
Langdon, K, Warne, MStJ & Sunderam, RIM, 2009, A compilation of data on the toxicity of chemicals to species in Australasia. Part 4: Metals (20022009), Australasian Journal of Ecotoxicology, vol. 15, pp. 51184.
Lexmond, TM 1980, The effect of soil-pH on copper toxicity to forage maize grown under field conditions’, Netherlands Journal of Agricultural Science, vol. 28, pp. 164183.
Lexmond, TM, Edelman T & van Driel, W 1986, ‘Voorlopige referentiewaarden en huidige achtergrondgehalten voor een antal zware metalen en arseen in de bovengrond van natuurterreinen en landbouwgronden’, Advies Bodemkwaliteit, VTCB A86/02, Ministerie van Volkshuisvesting, Ruimtelijke Ordening en Milieubeheer, Leidschendam.
Li, F, Okazaki, M & Zhou, Q 2003, ‘Evaluation of Cd uptake by plants estimated from total Cd, pH and organic matter’, Bulletin of Environmental Contamination and Toxicology, vol. 71, pp. 714721.
Lock, K & Janssen, CR 2001, ‘Modelling zinc toxicity for terrestrial invertebrates’, Environmental Toxicology and Chemistry, vol. 9, pp. 19011908.
Luoma, SN & Rainbow, PS 2008, Metal contamination in aquatic environments, Cambridge University Press, Cambridge, UK.
Maltby, L, Blake, N, Brock, TCM & van den Brink, PJ 2003, Addressing interspecific variation in sensitivity and the potential to reduce this source of uncertainty in ecotoxicological assessments, Science and Research Report PN0932, Department for Environment, Food and Rural Affairs (DEFRA), London, UK. (Available online at www.defra.gov.uk/science.
Maltby, L, Blake, N, Brock, TCM & van den Brink, PJ 2005, ‘Insecticide species sensitivity distributions: importance of test species selection and relevance to aquatic ecosystems’, Environmental Toxicology and Chemistry, vol. 24, no. 2, pp. 379388.
Markich, SJ & Camilleri, C 1997, ‘Investigation of metal toxicity to tropical biota: recommendations for revision of the Australian water quality guidelines, Report Number 127, Supervising Scientist, Canberra, Australia.
Markich, SJ, Warne, MStJ, Westbury, A-M and Roberts, CJ 2002, A compilation of data on the toxicity of chemicals to species in Australia, part 3: metals‘, Australasian Journal of Ecotoxicology, vol. 8, pp. 1138.
Marschner, P, Rengel, Z 2007, Nutrient cycling in terrestrial ecosystems, Springer Verlag, Berlin, Heidelberg.
McBride, MB 1989, ‘Reactions controlling heavy metal solubility in soils‘, Advances in Agronomy, vol. 10, pp. 156.
McLaughlin, MJ, Hamon, RE, McLaren, RG, Speir, TW & Rogers, SL 2000a, ‘Review: a bioavailability-based rationale for controlling metal and metalloid contamination of agricultural land in Australia and New Zealand‘, Australian Journal of Soil Research, vol. 38, pp. 10371086.
McLaughlin, MJ, Stevens, DP, Zarcinas, BA & Cook, N 2000b, ‘Soil testing for heavy metals’, Communications in Soil Science and Plant Analysis, vol. 31, pp. 16611700.
McLaughlin, MJ & Smolders, E 2001, ‘Background concentrations in soil affect the zinc sensitivity of soil microbial processes – A rationale for a metalloregion approach to risk assessments’, Environmental Toxicology and Chemistry, vol 20, pp. 26392643.
McLaughlin, MJ, Whatmuff, M, Warne, MStJ, Heemsbergen, D, Barry, G, Bell, M, Nash, D & Pritchard, D 2006, ‘A field investigation of solubility and food chain accumulation of biosolid-cadmium across diverse soil types’, Environmental Chemistry, vol. 3, pp. 428432.
McLaughlin, MJ, Lofts, S, Warne, MStJ, Amorim, AJM, Fairbrother, A, Lanno, R, Hendershot, W, Schlekat, CE, Ma, Y & Paton GI 2010, ‘Derivation of ecologically-based soil standards for trace elements’, in Merrington, G and Schoeters, I (eds), Soil Quality Standards for Trace Elements: Derivation, Implementation And Interpretation, pp. 780, Society of Environmental Toxicology and Chemistry (SETAC) jointly published with Taylor and Francis, Pensacola, Florida, USA, ISBN 978-1-4398-3023-9.
Menzies, NW, Donn, MJ & Kopittke PM, 2007, ‘Evaluation of extractants for estimation of the phytoavailable trace metals in soils’, Environmental Pollution vol. 145, pp. 121–130.
Ministry for the Environment 1996, A proposed methodology for deriving aquatic guideline values for toxic contaminants document for public comment, New Zealand Ministry of the Environment.
Moore, DRJ & Caux, PY 1997, ‘Estimating low toxic effects’, Environmental Toxicology and Chemistry, vol. 16, pp. 794801.
Morrissey, CA, Bendell-Young, LI & Elliott, JE 2005, ‘Identifying sources and biomagnification of persistent organic contaminants in biota from mountain streams of south-western British Columbia’, Canada Environmental Science and Technology, vol. 39, pp. 80908098.
Morton, R, Warne, MStJ & Correll, RL 2008, ‘Simultaneous prediction of toxicity of multiple chemicals to multiple species using multivariate functional relationships’, Environmetrics, vol. 19, pp. 765784.
Muller, J, Muller, R, Goudkamp, K, Shaw, M, Mortimer, M, Haynes, D et al. 2004, Dioxins in soils in Australia, National Dioxins Program, Technical report no. 5, Department of the Environment and Heritage, Canberra, Australia.
Newman, MC, Ownby, DR, Mezin, LCA, Powell, DC, Christensen, TRL, Lerberg, SB et al. 2000, ‘Applying species sensitivity distributions in ecological risk assessment: assumptions of distribution type and sufficient number of species’, Environmental Toxicology and Chemistry, vol. 19, pp. 508515.
Newman, MC 2008, ‘What exactly are you inferring? A closer look at hypothesis testing‘, Environmental Toxicology and Chemistry, vol. 27, pp. 10131019.
Nicholson, BC 1984, Water quality criteria for organic compounds, Australian Water Resources Council, Technical paper 82, Australian Government Publishing Service, Canberra, Australia.
Nolan, AL, Zhang, H & McLaughlin MJ 2005, ‘Prediction of zinc, cadmium, lead, and copper availability to wheat in contaminated soils using chemical speciation, diffusive gradients in thin films, extraction, and isotopic dilution techniques’, Journal of Environmental Quality, vol. 34, pp. 496–507.
OECD 1992, Report of the OECD workshop on extrapolation of laboratory aquatic toxicity data to the real environment, OECD Environment monographs, no. 59, Organisation for Economic Cooperation and Development, Paris, France.
OECD 1994, Draft guidance document for aquatic effects assessment, OECD Environment Monographs, no. 92, Organisation for Economic Cooperation and Development, Paris, France.
OECD 1995, Guidance document for aquatic effects assessment, OECD Environment Monographs, no. 92, Organisation for Economic Cooperation and Development, Paris, France.
Okkerman, PC, van de Plassche, EJ, Emans, HJB & Canton JH 1993, ‘Validation of some extrapolation methods with toxicity data derived from multiple species experiments‘, Ecotoxicology Environmental Safety, vol. 25, pp. 341-359.
Okkerman, PC, van de Plassche, EJ, Slooff, W, van Leeuwen, CJ & Canton, JH 1991, ‘Ecotoxicological effects assessment: a comparison of several extrapolation procedures‘, Ecotoxicology Environmental Safety, vol. 21, pp. 182191.
Olszowy, H, Torr, P, Imray, P, Smith, P, Hegarty, J & Hastie, G 1995, Trace element concentrations in soils from rural and urban areas of Australia, Contaminated Sites monograph no. 4, South Australian Health Commission, Adelaide, Australia.
Oorts, K, Ghesquiere, U, Swinnen, K & Smolders, E 2006, ‘Soil properties affecting the toxicity of CuCl2 and NiCl2 for soil microbial processes in freshly spiked soils’, Environmental Toxicology and Chemistry, vol. 25, pp. 836844.
Pedersen, F, Kristensen, P, Damborg, A & Christensen, HW 1994, ‘Ecotoxicological evaluation of industrial wastewater‘, Miljøprojekt no. 254, Ministry of Environment, Copenhagen, Denmark.
Pennington, DW 2003, ‘Extrapolating ecotoxicological measures from small data sets‘, Ecotoxicology Environmental Safety, vol. 56, pp 238250.
Posthumus, R & Slooff, W 2001, Implementation of QSARs in ecotoxicological risk assessment, RIVM report 601516003, Bilthoven, Netherlands.
Posthuma, L 1997, ‘Effects of toxicants on population and community parameters in field conditions, and their potential use in the validation of risk assessment methods’, Ecological Risk Assessment of Contaminants in Soils, vol 5, pp.85123.
Posthuma, L, Notenboom, J 1996, Toxic effects of heavy metals in three worm species exposed in artificially contaminated soil substrates and contaminated field soils, Bilthoven: RIVM. Report no. 719102048, 79pp.
Posthuma, L, Suter, GW II & Traas, T 2002, Species sensitivity distributions in ecotoxicology, Lewis Publishers, Boca Raton, Florida, USA.
Raimondo, S, Vivian, DN & Barron, MG 2007, Web-based interspecies correlation estimation (Web-ICE) for acute toxicity: user manual, version 2, EPA/600/R-07/071, US EPA, Office of Research and Development, National Health and Environmental Effects Research Laboratory, Gulf Ecology Division, Gulf Breeze, FL 32561, USA.
Rand, GM, Wells, PG & McCarty, LS 1995, ‘Introduction to aquatic toxicology’, in Rand, GM (ed.) Fundamentals of aquatic toxicology: effects, environmental fate and risk assessment, pp. 367, 2nd edn, Taylor and Francis, Washington DC, USA.
Reimann, C & Garrett, RG 2005, ‘Geochemical background — concept and reality’,Science of the Total Environment, vol. 350, pp. 1227.
Romijn, CAFM, Luttik, R, Slooff, W & Canton, JH 1991, Presentation of a general algorithm for effect-assessment on secondary poisoning, II, Terrestrial food chains, Report no. 679102007, National Institute of Public Health and Environmental Protection, Bilthoven, Netherlands.
Rooney, C, Zhao, FJ, McGrath, SP 2006, ‘Soil factors controlling the expression of copper toxicity to plants in a wide range of European soils‘, Environmental Toxicology and Chemistry, vol. 25, pp. 726732.
Roux, DJ, Jooste, SHJ & MacKay, HM 1996, ‘Substance-specific water quality criteria for the protection of South African freshwater ecosystems: methods for the derivation and initial results for some inorganic toxic substances, South African Journal of Science, vol. 92, pp. 198206.
Rusk, JA, Hamon, RE, Stevens, DP & McLaughlin MJ 2004, ‘Adaption of soil biological nitrification to heavy metals’, Environmental Science and Technology, vol 38, pp.30922097.
Rutgers, M, Sweegers, BMC, Wind, BS, van Veen, RPM, Folkerts, AJ, Posthuma L & Breure, AM 1998, ‘Pollution induced community tolerance in terrestrial microbial communities’, Contaminated Soil, vol. 1 & 2, pp. 337343.
Schudoma, D 1994, ‘Derivation of water quality objectives for hazardous substances to protect aquatic ecosystems: single-species test approach’, Environmental Toxicology and Water Quality, vol. 9, pp. 263272.
Shao, Q 2000, ‘Estimation for hazardous concentrations based on NOEC data: an alternative approach’, Envirometrics, vol. 11, pp. 583595.
Simpson, SL, Batley, G, Chariton, A, Stauber, JL, King, CK, Chapman, JC et al. 2005, Handbook for sediment quality assessment, Centre for Environmental Contaminants Research, CSIRO, Bangor, NSW, available online at http://www.clw.csiro.au/cecr/sedimenthandbook.
Simpson, SL & Batley, GE 2007, Revision of the ANZECC/ARMCANZ sediment quality guidelines, CLW science report, prepared for the Department of Agriculture, Fisheries and Forestry, Sydney, Australia.
Smith, EP & Cairns, JJr 1993, ‘Extrapolation methods for setting ecological standards for water quality: statistical and ecological concerns‘, Ecotoxicology, vol. 2, pp. 203219.
Smolders, E, Buekers, J, Waegeneers, N, Oliver, I & McLaughlin, MJ 2003, Effects of field and laboratory Zn contamination on soil microbial processes and plant growth, Final report to the International Lead and Zinc Research Organisation (ILZRO), Katholieke Universiteit Leuven & CSIRO.
Smolders, E, Buekers, J, Oliver, I & McLaughlin, MJ 2004, ‘Soil properties affecting toxicity of zinc to soil microbial properties in laboratory-spiked and field-contaminated soils’, Environmental Toxicology Chemistry, vol. 23, pp. 26332640.
Smolders, E & Degryse, F 2007, ‘Fixation of cadmium and zinc in soils: implications for risk assessment’, in Hamon, R, McLaughlin, M & Lombi, E (eds), Natural attenuation of trace element availability in soils, pp. 157171, SETAC Press, Florida.
Smolders, E, Oorts, K, van Sprang, P, Schoeters, I, Janssen, C, McGrath, S & McLaughlin, M 2009, ‘Toxicity of trace metals in soil as affected by soil type and aging after contamination using calibrated bioavailability models to set ecological soil standards’, Environmental Toxicology and Chemistry, vol. 28, pp. 16331642.
Song, J, Zhao, F-J, McGrath, SP & Luo, Y-M 2006, ‘Influence of soil properties and aging on arsenic phytotoxicity’, Environmental Toxicology Chemistry, vol. 25, pp. 16631670.
Staudinger, J & Roberts, PV 1996, ‘A critical review of Henry's law constants for environmental applications’, Critical Reviews in Environmental Science and Technology, vol. 26, pp. 205297.
Stephan, CE, Mount, DI, Hansen, DJ, Gentile, JH, Chapman, GA & Brungs, WA 1985, Guidelines for deriving numerical national water quality criteria for the protection of aquatic organisms and their uses, EPA/PB-85/227049, Washington, DC, USA.
Stevens, DP, McLaughlin, MJ, & Heinrich, T 2003, ‘Determining toxicity of lead and zinc runoff in soils: salinity effects on metal partitioning and on phytotoxicity’, Environmental Toxicology Chemistry, vol. 22, pp. 30173024.
Stokes, JD, Paton, GI, Semple, KT 2005, ‘Behaviour and assessment of bioavailability of organic contaminants in soil: relevance for risk assessment and remediation‘, Soil Use and Management, vol. 21, pp. 475486.
Struijs, J, van den Meent, D, Peijnenburg, WJGM, van den Hoop, MAGT & Crommentuijn, T 1997, ‘Added risk approach to derived maximum permissible concentrations of heavy metals: How to take natural background levels into account’, Ecotoxicology and Environmental Safety, vol. 37, pp. 112118.
Taylor, RM 1983, Soils: An Australian perspective, sponsored by the Division of Soils, Commonwelath Scientific and Industrial Research Organisation, Australia (CSIRO), Academic Press, London, UK.
Traas, TP 2001, Guidance document on deriving environmental risk limits, RIVM report number 601501 012. Rijks Instituut Voor Volksgezondheid En Milieu, Bilthoven, Netherlands.
US EPA 1986, Quality criteria for water, EPA 440/5-86/001, US Environmental Protection Agency, US Department of Commerce, Virginia, USA.
US EPA 1991, Technical support document for water quality-based toxics control, EPA 505/2-90/001, US Environmental Protection Agency, US Department of Commerce, Virginia, USA.
US EPA 1994, AQUIRE (Aquatic toxicity information retrieval), Office of Research and Development, National Health and Environmental Effects Research Laboratory, Mid-Contintental Ecology Division, Duluth, Minnesota, USA.
US EPA 1996, Soil screening guidance: technical background, EPA/540/R-95/128, US Environmental Protection Agency, US Department of Commerce, Virginia, USA.
US EPA 1999, Water quality guidance for the Great Lakes system, Federal register, 40 CFR, part 142, July 1, US Environmental Protection Agency, US Department of Commerce, Virginia, USA.
US EPA 2004, Ecotox database, US Environmental Protection Authority, available online at www.epa.gov/ecotox.
van Beelen, P, Verbruggen, EMJ & Peijnenburg, WJGM 2003, ‘The evaluation of the equilibiurm partitioning method using species sensitivity distributions of species in water and soil’, Chemosphere, vol. 52, pp. 11531162.
van de Plassche, EJ, Polder, MD & Canton, JH 1993, Derivation of maximum permissible concentrations for several volatile compounds for water and soil, Report no. 679101008, National Institute of Public Health and Environment Protection, Bilthoven, Netherlands.
van de Plassche, EJ 1994, Towards integrated environmental quality objectives for several compounds with a potential for secondary poisoning, Report no. 679101012, National Institute of Public Health and Environment Protection, Bilthoven, Netherlands.
van Gestel, CAM, Ma, WC & Smit, CE 1991, ‘Development of QSARs in terrestrial ecotoxicology: earthworm toxicity and soil sorption of cholorophenols, chlorobenzenes and dichloroaniline’, Science of the Total Environment, vol. 109110, pp. 589604.
van Gestel, CAM 1992, The influence of soil characteristics on the toxicity of chemicals for earthworms: a review, in Becker, H (ed.), Ecotoxicology of earthworms, pp. 4454, Intercept, Andover, UK.
van Straalen, NM & Denneman, CAJ 1989, ‘Ecotoxicological evaluation of soil quality criteria’, Ecotoxicology Environmental Safety, vol. 18, pp. 241251.
van Vlaardingen, PLA & Verbruggen, EMJ 2007, Guidance for the derivation of environmental risk limits within the framework of the project “International and National Environmental Quality Standards in the Netherlands”, RIVM report number 601501 031, National Institute for Public Health and the Environment (RIVM), Bilthoven, Netherlands.
Vijver, M, Jager, T, Posthuma, L, & Peijnenburg, W 2001, ‘Impact of metal pools and soil properties on metal accumulation in Folsomia candida (Collembola), Environmental Toxicology and Chemistry, vol. 20, pp. 712–720.
VLAREBO 2008, Flemish soil remediation decree, Vlaams Reglement Bodemsanering ratified 14 December 2007, published 22 April.
VROM 2000, Circular on target values and intervention values for soil remediation, reference DBO/1999226863, Ministry of Housing, Spatial Planning and the Environment, Bilthoven, Netherlands.
Wagner, C & Løkke, H 1991, ‘Estimation of ecotoxicology protection levels from NOEC toxicity data’, Water Research, vol. 25, pp. 12371242.
Warne, MStJ 1996, ‘The theory and practice of extrapolation techniques to derive environmental quality criteria: the Dutch approach‘, in Proceedings of the third national workshop on the health risk assessment and management of contaminated sites, pp. 402416.
Warne, MStJ 1998, ‘Critical review of methods to derive water quality guidelines for toxicants and a proposal for a new framework’, Supervising Scientist Report 135, Supervising Scientist, Canberra, Australia.
Warne, MStJ, Westbury, A-M & Sunderam, RIM 1998, ‘A compilation of data on the toxicity of chemicals to species in Australasia, part 1: pesticides’, Australasian Journal of Ecotoxicology, vol. 4, pp. 93144.
Warne, MStJ, Westbury, A-M 1999, ‘A compilation of toxicity data for chemicals to Australasian species, part II: organic chemicals’, Australasian Journal of Ecotoxicology, vol. 5, pp. 2185.
Warne, MStJ 2001, ‘Derivation of the ANZECC and ARMCANZ water quality guidelines for toxicants, Australasian Journal of Ecotoxicology, vol.7, pp. 123136.
Warne, MStJ, Palmer, CG & Muller, WJ, 2004a, Water quality guideline development programme (WQGD), development of pilot guidelines for selected organic toxicants/toxicity effects,I. Protocol for aquatic ecosystem guideline development, Client report to the South African Department of Department of Water Affairs and Forestry.
Warne, MStJ, Palmer, CG & Muller, WJ, 2004b, Water quality guideline development programme (WQGD), development of pilot guidelines for selected organic toxicants/toxicity effects, II. Protocol application to DDT, Client report to the South African Department of Department of Water Affairs and Forestry.
Warne, MStJ, McLaughlin, MJ, Heemsbergen, DA, Bell, M, Broos, K, Whatmuff, M et al. 2007, Draft position paper: recommendations of the Australian national biosolids research program on biosolids guidelines, Adelaide, South Australia, available online at http://www.clw.csiro.au/research/biogeochemistry/organics/biosolids/positionpaper.html.
Warne, MStJ & Van Dam, R 2008, ‘NOEC and LOEC data should no longer be generated or used’, Australasian Journal of Ecotoxicology, vol. 14, no. 1, pp. 15.
Warne, MStJ, Heemsbergen, DA, Stevens, D, McLaughlin, MJ, Cozens, G, Whatmuff, M et al. 2008a, ‘Modelling the toxicity of Cu and Zn salts to wheat in fourteen soils’, Environmental Toxicology and Chemistry, vol. 27, pp. 786792.
Warne, MStJ, Heemsbergen, DA, McLaughlin, MJ, Bell, M, Broos, K, Whatmuff, M, Barry, G, Nash, D, Pritchard, D & Penney, N 2008b, ‘Models for the field-based toxicity of copper and zinc salts to wheat in eleven Australian soils and comparison to laboratory-based models’, Environmental Pollution, vol. 156, no. 3, pp. 707714.
Westbury, A-M, Warne, MStJ & Lim, RP 2004, ‘Toxicity of substituted phenols to Ceriodaphnia cf. dubia and Vibrio fischeri and the development of predictive models’, Australasian Journal of Ecotoxicology, vol. 10, pp. 3342.
Wheeler, JR, Grist, EPM, Leung, KMY, Morritt, D & Crane, M 2002, ‘Species sensitivity distributions: data and model choice‘, Marine Pollution Bulletin, vol. 45, pp.192202.
Wilson, SC, Duarte-Davidson, R & Jones, KC 1996, ‘Screening the environmental fate of organic contaminants in sewage sludges applied to agricultural soils: 1. the potential for downward movement to groundwaters’, Science of the Total Environment, vol. 185, pp. 4557.
Zhao, F-J, Rooney, CP, Zhang, H & McGrath, SP 2006, ‘Comparison of soil solution speciation and diffusive gradients in thin films measurement as an indicator of copper bioavailability to plants’, Environmental Toxicology and Chemistry, vol. 25, pp. 733742.
Zhao, FJ, McGrath, SP & Merrington, G, 2007, ‘Estimates of ambient background concentrations of trace metals in soils for risk assessment’, Environmental Pollution, vol. 148, pp. 221229.
Further Reading
ANZECC & NHMRC 1992, Australian and New Zealand guidelines for the assessment and management of contaminated sites, Australian and New Zealand Environment and Conservation Council & National Health and Medical Research Council.
Bakker, J, Brandes, LJ, Den Hollander, HA, van de Meent, D & Struijs, J 2003, Validating simple box-computed steady-state concentration ratios, Report no. 607220010/2003, National Institute of Public Health and Environmental Protection, Bilthoven, Netherlands.
Checkai, RT, Corey, RB & Helmke, PA 1987, ‘Effects of ionic and complexed metal concentrations on plant uptake of cadmium and micronutrient metals from solution’, Plant and Soil, vol. 99, pp. 335345.
DPIWE 1999, Tasmanian biosolids re-use guidelines, Department of Primary Industry, Water and Environment, Hobart, Tasmania, Australia.
EPA Vic 2004, Guidelines for environmental management, biosolids land application, Publication 943, Environmental Protection Agency Victoria, Melbourne, Australia.
Kefford, BJ, Palmer, CG, Jooste, S, Warne, MStJ & Nugegoda, D 2005, ‘What is meant by 95% of species? An argument for the inclusion of rapid tolerance testing‘, Human and Ecological Risk Assessment, vol. 11, pp. 10251046.
Laurie, SH & Manthey, JA 1994, ‘The chemistry and role of metal ion chelation in plant uptake processes‘, in Manthey, JA, Crowley, DE & Luster, DG (eds), Biochemistry of metal micronutrients in the rhizoshpere, pp. 165182, Lewis Publishers, Boca Raton, Florida, USA.
Ministry for the Environment 2003, Contaminated land management guidelines no. 2, Hierarchy and application in New Zealand of environmental guideline values, Ministry for the Environment, Wellington, New Zealand.
Minnich, MM, McBride, MB & Chaney, RL 1987, ‘Copper activity in soil solution: 2, relation to copper accumulation in young snapbeans’, Soil Science Society of America Journal, vol. 51, pp. 573578.
Nan, Z, Zhao, C, Li, J, Chen, F & Sun, W 2002, ‘Relations between soil characteristics and selected heavy metal concentrations in spring wheat (Triticum aestivum L.) grown in contaminated soils‘, Water Air and Soil Pollution, vol. 133, pp. 205213.
NRMCC 2004, Guidelines for sewerage systems – biosolids management, National water quality management strategy, Paper 13, National Resource Management Ministers Council, Canberra, Australia.
NEPC 1999, ‘Schedule B(5), Guideline on ecological risk assessment’, National Environment Protection (Assessment of Site Contamination) Measure 1999, National Environment Protection Council , Adelaide, Australia.
NEPC 1999, ‘Schedule B(1), Guideline on the investigation levels for soil and groundwater’, National Environment Protection (Assessment of Site Contamination) Measure 1999, National Environment Protection Council, Adelaide, Australia.
NEPC 2005, Review of the National Environment Protection (Assessment of Site Contamination) Measure: issues paper, National Environment Protection Council, Adelaide, Australia.
NEPC 2006a, National Environment Protection (Assessment of Site Contamination) Measure: NEPM Review discussion paper, National Environment Protection Council, Adelaide, Australia.
NEPC 2006b, National Environment Protection (Assessment of Site Contamination) Measure Review: Review report, National Environment Protection Council, Adelaide, Australia.
NSW EPA 1997, Environmental guidelines: use and disposal of biosolids products, New South Wales Environmental Protection Authority, Sydney, Australia.
NSW DEC 2006, Contaminated sites: guidelines for the NSW site auditor scheme (2nd edition), New South Wales Department of Environment and Conservation, Sydney, Australia.
OECD 1984, ‘Earthworm acute toxicity tests’, OECD Guideline for testing of chemicals, 207, Organisation for Economic Cooperation and Development, Paris, France.
Petersen, LS & Pedersen, F 1995, Water quality criteria for selected priority substances, Danish Ministry of the Environment and Energy, Danish Environmental Protection Agency, Copenhagen, Denmark.
Piwoni, MD & Banerjee, P 1989, ‘Sorption of volatile organic solvents from aqueous solution onto subsurface solids’, Journal of Contaminant Hydrology, vol. 4, pp. 163179.
SA EPA 1997, South Australian biosolids guidelines for the safe handling, re-use or disposal of biosolids, South Australian Environment Protection Authority, South Australian Department of Environment and Natural Resources, Adelaide, Australia.
van de Meent, D & de Bruijn, JHM 1995, ‘A modelling procedure to evaluate the coherence of independently derived environmental quality objectives for air, water and soil’, Environmental Toxicology and Chemistry, vol. 14, pp. 177186.
WA DEP 2002, Western Australian guidelines for direct application of biosolids and biosolid products, Western Australia Department of Environmental Protection, Perth, Australia.
The US EPA has developed a series of Eco-SSLs (www.epa.gov/ecotox/ecossl/) to protect terrestrial organisms from soil contamination.
Eco-SSLs apply to sites where terrestrial organisms may be exposed directly or indirectly to contaminated soil. Eco-SSLs were developed to support risk management decisions for Superfund sites (orphaned contaminated sites identified as having significant contamination potentially present for many years or even decades). This was undertaken to avoid repetitious risk assessment and literature reviews of toxicity data for the same contaminants at each contaminated site, and to allow risk assessors to focus their efforts on the main contaminants of concern.
Seven types of receptors were initially considered in the development of the Eco-SSLs (mammals, birds, reptiles, amphibians, soil invertebrates, plants, and soil microbes and their processes) but final SSLs were produced without consideration of amphibians and reptiles due to insufficient data being available, in the view of the US EPA, to derive screening levels. Soil microorganisms and microbial processes were also not included in the derivation of Eco-SSLs but the rationale for this was over the variability of the data and their ecological significance.
For plants and invertebrates, the methodology used to develop Eco-SSLs was to review the relevant toxicity literature for each contaminant, screen the data for quality, and only use toxicity data representing high bioavailability conditions in upland aerobic soils (that is, avoiding consideration of flooded soil conditions). Because of the different behaviour of many contaminants in soils, high bioavailability was defined for three broad groups of contaminants—cationic metals, anionic metals and non-ionising organic contaminants. For example, high bioavailability for cationic metals was defined as low soil pH and organic matter content. Where literature data did not exist for a contaminant, this was developed by experimentation.
The Eco-SSL for a contaminant was calculated as the geometric mean of all the toxicity values at the highest relative bioavailability score for which sufficient data existed (that is, ≥3 data points). If less than three data values were available at the highest relative bioavailability level, data from the next highest bioavailability score was included in that Eco-SSL data set. This process proceeded until a combined data set of three or more data values was identified for calculating the Eco-SSL. If there were less than three acceptable studies, an Eco-SSL was not calculated.
For wildlife Eco-SSLs, three avian and three mammalian species were chosen to represent some of the most highly exposed species at contaminated sites (meadow vole, short-tailed shrew, long-tailed weasel, mourning dove, American woodcock and red-tailed hawk). Wildlife Eco-SSLs were developed by back-calculating from a hazard quotient (HQ) of 1.0, calculated by dividing the estimated exposure dose by the toxicity reference value (TRV). When the HQ was 1.0, the exposure dose equalled the Eco-SSL.
A generic food-chain model was used to estimate the relationship between the concentration of the contaminant in soil and the critical dose (TRV). TRVs were developed using a literature screening process similar to that of the plant and invertebrate Eco-SSLs.
Twenty-four Eco-SSLs have been produced for aluminium, antimony, arsenic, barium, beryllium, cadmium, chromium, cobalt, copper, iron, lead, manganese, nickel, selenium, silver, vanadium, zinc, dieldrin, hexahydro -1,3,5-trinitro-1,3,5-triazine (RDX), trinitrotoluene (TNT), dichloro-diphenyl-trichloroethane (DDT) and its metabolites (DDE and DDD), pentachlorophenol, PAHs, and polychlorinated biphenyls (PCBs).
As part of the Dutch Soil Protection Act (VROM 2000), the Netherlands Ministry of Housing, Spatial Planning and the Environment (VROM) has developed a series of soil-screening values for contaminated sites, remediation and long-term soil concentration goals, based on protection of soil health.
Soil quality is assessed and managed using three soil screening values—the target and intervention value and a value between these two termed the intermediate value. These values are independent of land use. Soils with contaminant concentrations below target value are considered to be at no risk and no restrictions on their use have been set. Soils with contaminant concentrations below the intermediate values can have certain restrictions set on soil and site management. Soils with contaminant concentrations exceeding intermediate but below the intervention value require further investigation of the site to assess the hazard posed by the contaminants. Soils with contaminant concentrations exceeding the intervention value require remediation as a matter of urgency.
Remediation levels for contaminants in soils have a separate set of values, the so-called reference values. These values are land use-specific, but site-specific reference values can be derived. Land uses are grouped into four clusters: 1) residential and intensively used parkland, 2) extensively used parkland, 3) buildings and paved areas, and 4) agriculture and nature reserves.
The intervention and target values are preferably derived using an SSD method with a log-normal distribution. Toxicity data used in the SSD approach are NOECs and LOECs but if these are not available, higher adverse effect data is used and converted to NOECs using a safety factor of 10. Toxicity data is normalised to a reference soil of 10% organic matter and 25% clay. The equations used to normalise the toxicity data (that is, normalisation equations) are based on the studies by Lexmond et al. (1986) and Van Straalen and Denneman (1989), where background levels of contaminants showed a positive relationship with organic matter and/or clay. Intervention values are designed to protect 50% of the species. In other words, the permitted concentration is hazardous to 50% of species and hence referred to as the HC50. Target values are equal to the HC5 (that is, the concentration that should permit only 5% of species to be affected) divided by 100. This factor 100 is applied to take into account combination toxicity (Crommentuijn 2000a).
If limited toxicity data is available, equilibrium partitioning (EqP) methods are used to derive soil screening values by extrapolation of aquatic toxicity data. If no data is available, the Dutch guidelines use QSARs to estimate toxicity data from contaminants that have the same mechanism of action.
Intervention and target values have been set for 75 contaminants and a further 20 contaminants have target values and/or indicative levels of serious contaminant levels (VROM 2000).
The Canadian SQGs were developed by CCME to assess in-place contaminants in soil (CCME 1999, 2006) and can be found at: www.ccme.ca/publications/list_publications.html#link2.
SSQs and the level of protection for terrestrial species and soil processes depend on land use (that is, agriculture, residential/parkland, commercial and industrial sites). Using potential exposure scenarios, ecological receptors that sustain the primary activities for each land use are identified. These include soil invertebrates, soil nutrient cycling processes, plants, wildlife for all four land uses, soil and food ingestion by herbivores and consumers (including biomagnification) for residential and agricultural, and crops and livestock for agricultural land use.
SSQs were derived using laboratory and field-based toxicity data. This data measures the effects that undermine a species' ability to survive and reproduce under normal living conditions for soils that represented typical Canadian soils. The preferred measures of toxicity are 25% effect concentrations (IC25 or EC25). A second option is to use LOECs divided by an uncertainty factor (safety factor) if there is insufficient 25% effect data (SSD method). A third option is to use median effect data (LC50 or EC50) divided by an uncertainty factor (for agricultural and residential/parkland only, not for commercial and industrial sites). Depending on the quantity of toxicity data available, the weight-of-evidence (SSD) approach, LOEC method or median effects method was used to obtain SQGs. SSD was the preferred methodology if sufficient data was available. The output from the SSD might be divided by an uncertainty factor, depending on the type and amount of toxicity data used in the SSD. For the agricultural and residential/parkland land uses, the SQGs derived using an SSD (IC25 and/or EC25 data) are set to protect 75% of species and soil processes while, for commercial and industry land uses, 50% of the species are protected. A full description of the methodology can be found online at www.ccme.ca/assets/pdf/sg_protocol_1332_e.pdf.
If sufficient toxicity data is available, the SQGs distinguish between two generic soil types: coarse-textured soils (soils containing predominantly sand and gravel) and fine-textured soils (soils containing predominantly silt and clay). This separation has been made as contaminant fate, transport and bioavailability are dependent to varying degrees on soil texture, moisture content and other factors. Separation of the two soil types can thereby minimise the uncertainty in guideline derivation introduced by soil variability.
Thirty-two SQGs have been produced using the 1999 or 2006 derivation protocol, and 34 interim remediation criteria in soils remain (established in 1991) that have not yet been replaced by the SQG protocol. A complete list of SSQs and interim remediation criteria can be viewed at www.documents.ccme.ca.
The SQGs include: arsenic (inorganic), barium, benzene, benzo(a)pyrene, cadmium, chromium (total and Cr VI), copper, cyanide (free), DDT (total), di-isopropanolamine, ethylbenzene, ethylene glycol, lead, mercury (inorganic), naphthalene, nickel, nonylphenol (and its ethyloxylates), pentachlorophenol, phenol, PCBs, polychlorinated dibenzo-p-dioxins/dibenzofurans (PCDD/Fs), propylene glycol, selenium sulfolane, tetrachloroethylene, thallium, toluene, trichloroethylene, uranium, vanadium, xylenes, and zinc.
The interim remediation criteria include: conductivity, pH, sodium adsorption ratio, antimony, beryllium, boron (hot water soluble), cobalt, fluoride (total), molybdenum, silver, sulfur (elemental), tin, chlorobenzene, 1,2-dichlorobenzene, 1,3-dichlorobenzene, 1,4-dichlorobenzene, styrene, chlorophenols, nonchlorinated phenolic compounds, benzo(a)anthtracene, benzo(b)fluoranthene, benzo(k)fluoranthene, dibenz(a,h)anthracene, indeno(1,2,3-c,d)pyrene, phenanthrene, pyrene, chlorinated aliphatics, chlorobenzenes, hexachlorobenzene, hexachlorocyclohexane, nonchlorinated aliphatics, phthalic acid esters, quinoline, and thiophene.
European Union Regulation 1488/94 and Directive 98/8 require that an environmental risk assessment be carried out on notified new substances, on priority existing substances and active substances and substances of concern in a biocidal product. Neither the regulation nor directive provides soil guideline values, but a technical guidance document (TGD) on ERA (ECB 2003) and soil guideline derivation was published as part of EU Directive 93/67 and is available online at
ecb.jrc.it/Documents/TECHNICAL_GUIDANCE_DOCUMENT/EDITION_2/tgdpart2_2ed.pdf.
Several member states, including the UK, have adopted the methodology for deriving their national SQGs given in the technical guidance document (ECB 2003). Eventually, all EU member states will develop SQGs and use the method recommended in the TGD (ECB 2003).
In the UK, soil guideline values (SGVs) represent ‘intervention values’ which, if exceeded, indicate potentially unacceptable risks to site users and therefore trigger further investigation. SGVs aim to be precautionary to ensure that all the potential sites of concern are captured at the screening stage.
The SGVs are derived by calculating a predicted no-effect concentration (PNEC) preferably using NOEC data or estimates of NOECs (larger effect toxicity data, for example, EC50, divided by a safety factor). The TGD (ECB 2003) recommends that, if possible, toxicity data should be normalised for the effect soil characteristics have on the toxicity of a contaminant.
The PNEC can be derived by three methodologies:
For the SSD, the TGD does not recommend a particular statistical distribution to be used in the SSD method. The output of the SSD is the HC5. Whether the HC5 value is protective is then assessed by the amount and type of toxicity data used in the SSD divided by an AF of between 1 and 5, depending on the uncertainties around the HC5.
Currently, the EU is performing environmental risk assessments on all the existing chemicals and these reports can be found online at www.ecb.jrc.it/.
An overview document is available for methodologies used for deriving soil screening values for individual European countries (Carlon 2007) and is available online at www.ies.jrc.cec.eu.int/fileadmin/Documentation/Reports/RWER/EUR_2006-2007/EUR22805-EN.pdf.
The German Federal Soil Protection and Contaminated Sites Ordinance (BBodSchV 1999) provides a series of precautionary, trigger and action values to protect terrestrial ecosystems from adverse effects from soil contamination. These values are used to prevent future soil contamination and for remediation of contaminated sites.
Precautionary values indicate a potential future soil impairment that should be averted. For inorganic chemicals, precautionary values are derived for three soil types: sandy, siltloam and clay soils. For organic chemicals, precautionary values are derived for two soil types: soils with a humus content >8% and with a humus content ≤8 %. The ordinance does not give guidance on how to derive precautionary values.
Once the precautionary values have been exceeded, the ordinance (BBodSchV 1999) provides additional annual loading limits of the contaminants to prevent the soil concentration reaching the trigger or action values and causing adverse effects.
Trigger values trigger the investigation of the contaminated site to ascertain if the contaminant poses a hazard. Action values represent a direct hazard situation which should be prevented and therefore soils exceeding action values should be remediated. Action and trigger values are land use-dependent and specific exposure pathways are assigned to each land use. Trigger and action values are developed for three exposure pathways: soil to human, soil to plant and soil to groundwater. Trigger values for inorganic contaminants and the soil to plant pathway are, if possible, based on an estimate of the bioavailable concentration (that is, measured in 1 M NH4NO3 soil extraction). The soil to plant values are based on regression analyses between soil and plant concentrations of the contaminant. A maximum internal plant concentration is set, either based on human health issues or plant toxicity, and the corresponding soil concentration, based on the linear regression, is the trigger or action value.
The New Zealand Ministry for the Environment has developed environmental guideline values (EGVs) for contaminated land assessment, which are available online at
www.mfe.govt.nz/publications/hazardous/contaminated-land-mgmt-guidelines-no2/
contaminated-land-mgmt-guidelines-no2.pdf. The contaminated land management guidelines are not regulations but a guideline to obtain the most appropriate EGVs for a contaminated site.
New Zealand EGVs contain values with some derived within New Zealand and others by international regulators (for example, Canada, the Netherlands, USA, Australia). Therefore, a suite of methods was used to derive these values. A distinction was made between risk-based and threshold-based EGVs which is based on quality and quantity of the data available and the method used to derive the values.
Risk-based values are derived from a given exposure scenario; for example, protection of human health or the protection of a nominal proportion of species in an ecosystem and thus are calculated using a SSD method.
Threshold values may be derived from toxicological data where insufficient data is available to calculate risk-based values. The EGVs may also be classified as threshold values where insufficient information on their derivation is provided.
A hierarchy was established to determine the order in which EGVs should be used in a contaminated site assessment. The hierarchy in descending order of use is:
Although EGVs are provided, the New Zealand framework stresses that the original reference document for an EGV must be referred to in order to assess if the EGV is relevant for the contaminated soil being investigated. Therefore, the EGVs and the framework are guidelines to obtain the most relevant EGV for a contaminated site.
The key physicochemical property of inorganic contaminants that controls their potential movement to ground and/or surface waters is the soilwater partition coefficient (Kd). This is the ratio of the concentration of a contaminant bound to the soil to that dissolved in soil pore water at equilibrium and therefore is related to the aqueous solubility of that contaminant. The lower the Kd, the more of a contaminant that will be present in the soil pore water. This may increase the potential for plants and soil invertebrates to be exposed via the pore water and increase the potential for leaching to groundwater and for groundwater organisms to be exposed. Although Kd is soil- and contaminant-dependent, a conservative cut-off point for inorganics at a log Kd of 3 is used in the methodology. The log Kd thresholds are presented in Table B1.
Table B1. Classification system used for the mobility of inorganic contaminants in soil, based on the logarithm of the soilwater partition coefficient (log Kd).
Log Kd value | Leachability |
<3 | High potential to leach (H) |
≥3 | Low potential to leach (L) |
For inorganics with a log Kd <3, leaching of the contaminant should be addressed if there is a water source in the immediate vicinity.
There are two partition coefficients related to the leaching potential of organic contaminants. The first is the octanolwater partition coefficient (Kow), that is, the ratio of the concentration of a contaminant that is dissolved in n-octanol to that dissolved in water at equilibrium and at a specified temperature. It is used as a surrogate to estimate the potential for contaminants to accumulate in tissue—both plant and animal (Connell 1989, Posthumus & Slooff 2001). The second is the organic carbonwater partition coefficient (Koc). Both Kow and Koc are chemical-specific values and collations of values for contaminants are widely available e.g. at http://www.epa.gov/region9/superfund/prg/.
Contaminants with a high log Koc preferentially partition to soil organic matter rather than water and thus have a low potential to leach. Conversely, contaminants with a low log Kow tend to have a high potential to leach. Log Kow and log Koc have a linear relationship (Briggs 1981, Connell 1989).
log Koc = 0.9 x log Kow + 0.62 (equation B1)
Therefore log Kow (which is much more readily available than log Koc) can act as a surrogate of the potential for contaminants to leach from soil to groundwater. On this basis, Wilson et al. (1996) used log Koc and log Kow to classify the mobility of organic contaminants in soil (Table B2).
Table B2. The classification system used for the mobility of organic contaminants in soil based on the logarithm of the organic carbonwater partition coefficient (log Koc) and logarithm of the octanolwater partition coefficient (log Kow). Modified from Wilson et al. 1996.
Corresponding log Kow values1 | log Koc | Classification of mobility |
<2 | <2.4 | Mobile (M) |
2.02.7 | 2.43.05 | Medium mobility (MM) |
2.73.7 | 3.053.95 | Low mobility (LM) |
>3.7 | >3.95 | Immobile (IMM) |
1. log Kow values corresponding to the log Koc values were derived using equation B1.
Many organic contaminants can degrade either biologically or chemically. Thus, it is recommended that EILs derived for organic contaminants with a slow degradation rate (that is, large half-life, refer to Table 2) and a log Koc (or log Kow) <4 should consider the protection of aquatic ecosystems where appropriate.
The US EPA methodology (US EPA 1996) may be used to calculate EILs that account for the potential of contaminants to leach and affect aquatic ecosystems. Although the method has its limitations due to several simplifications, it is a robust method where the required information is available for Australian soils.
The potential leaching of inorganic contaminants to the groundwater depends on the soil to water partitioning of the contaminant, Kd, which is contaminant- and soil-dependent. Furthermore, volatilisation can reduce the soil concentration of the inorganic contaminant and this amount will reduce the potential of the contaminant to leach to the groundwater. For essentially all inorganic contaminants, volatilisation is limited; however, for Hg, a substantial amount can be volatilised.
Because groundwater catchments will most likely contain both contaminated and uncontaminated soils, pore water concentrations of the contaminant in question will not always equal the groundwater concentration. Therefore, a dilution and attenuation factor (DAF) is used to take this into account. The fraction of contaminated land to the total area of the local groundwater/aquifer catchment can be used to calculate the DAF, as indicated by equation B1 below.
DAF = 100 ÷ percentage of contaminated soil in local catchment (equation B2)
Therefore, for inorganic contaminants the EIL is calculated as follows (US EPA 1996):
![]() (equation B3)
(equation B3)
where EIL is the ecological investigation level in soil (mg/kg), Cw is the target soil leachate concentration (mg/L) (that is, the appropriate WQG), Kd is the soil to water partition coefficient (L/kg), θw is the water-filled soil porosity Lwater/Lsoil), θa is the air-filled soil porosity (Lair/Lsoil), ρb is the dry soil bulk density (kg/L), H is the Henry’s law constant (unitless), and DAF is the dilution and attenuation factor.
Organic contaminants can bind to the organic carbon in soil. The extent of this depends on the properties of the contaminant and the amount and type of organic carbon in the soil. For organic contaminants the equation for soil to groundwater migration becomes (US EPA 1996):
![]() (equation B4)
(equation B4)
where EIL is the ecological investigation level in soil (mg/kg), Cw is the target soil leachate concentration (mg/L) (that is, the appropriate WQG), Koc is the organic carbon to water partition coefficient (L/kg), foc is the organic carbon content of soil (kg/kg), θw is the water-filled soil porosity (Lwater/Lsoil), θa is the air-filled soil porosity (Lair/Lsoil), ρb is the dry soil bulk density (kg/L), H is the Henry’s law constant (unitless), and DAF is the dilution and attenuation factor that is calculated as per equation B2.
The target soil leachate concentration (Cw) should be set as the relevant WQG for that contaminant in groundwater systems, which currently is the surface freshwater TV (ANZECC & ARMCANZ 2000).
The methodology for deriving EILs outlined in this Schedule accounts for the effects of soil reactions that modify the bioavailability of soluble contaminants. However, it does not take into account the form or bioavailability of the contaminant. The EIL derivation framework also makes the assumption that ecotoxicity data in the literature is derived using highly bioavailable forms of contaminants (for example, soluble metal salts or soluble organic molecules), and indeed this is generally the case for most ecotoxicity studies. Thus, the framework is reasonably conservative in its assumptions and protective, and is appropriate for a screening level risk assessment.
Soil contamination can occur from a variety of sources, and not all these sources have 100% bioavailability when they are initially added to soil; for example, vitreous slags, tyre debris, massive metal, encapsulated materials, etc.
When total concentrations of contaminants are determined in a soil containing these materials, these contaminants will be solubilised, assumed to be bioavailable, and therefore some sites may exceed the EILs, yet the actual risk is negligible. Further chemical investigation of the bioavailability of the contaminants should be undertaken prior to direct toxicity assessment.
For a detailed review of methods to assess metal bioavailability in soils, see McLaughlin et al. (2000b). For detailed reviews of methods to assess bioavailability of organic contaminants in soils see Stokes et al. (2005) and Dean and Scott (2004).
Information on leachability tests applicable to contaminated sites can be found in Schedule B3.
Adaptation is (1) change in an organism in response to changing conditions of the environment (specifically chemical), which occurs without any irreversible disruption of the given biological system and without exceeding the normal (homeostatic) capacities of its response, and (2) a process by which an organism stabilises its physiological condition after an environmental change. |
Added contaminant limit (ACL) is the added concentration of a contaminant above which further appropriate investigation and evaluation of the impact on ecological values will be required. ACL values are generated in the process of deriving ecological investigation levels (EILs). |
Adsorption is the adhesion of molecules to surfaces of solids. |
Ambient background concentration (ABC) of a contaminant is the soil concentration in a specified locality that is the sum of the naturally occurring background and the contaminant levels that have been introduced from diffuse or non-point sources by general anthropogenic activity not attributed to industrial, commercial, or agricultural activities. |
An area of ecological significance is one where the planning provisions or land use designation is for the primary intention of conserving and protecting the natural environment. This would include national parks, state parks, and wilderness areas and designated conservation areas. |
Bioaccumulation is the net result of the uptake, distribution and elimination of a substance due to all routes of exposure, that is, exposure to air, water, soil/sediment and food. |
Bioaccumulation factor is a partition coefficient for the distribution of a chemical between an organism exposed through all possible routes and an environmental compartment or food. |
Bioavailability is the ability of a contaminant to interact with the biological system of an organism. Not all of a contaminant that is present in environmental compartments (for example, soil, sediment, water and air) is biologically available—rather, only a fraction of the total (the bioavailable fraction) is available. |
Bioconcentration factor (BCF) is a quantitative measure of a chemical’s tendency to be taken up from the ambient environment (for example, water for aquatic organisms and soil or soil pore water for soil organisms). The BCF is the ratio of the concentration of the chemical in tissue (or a specific organ) and the concentration in the ambient environment. |
Bioconcentration is the net result of the uptake, distribution and elimination of a substance due to exposure in the ambient environment (for example, water for aquatic organisms and soil or soil pore water for soil organisms). |
Biological half life is the time needed to reduce the concentration of a test chemical in the environmental compartment or organisms to half the initial concentration, by transport processes, (for example, diffusive elimination), transformation processes (for example, biodegradation or metabolism) or growth. |
Biomagnification factor is the quantitative measure of a chemical’s tendency to be taken up through the food web. |
Biomagnification is the accumulation and transfer of chemicals via the food web due to ingestion, resulting in an increase of the internal concentration in organisms at the succeeding trophic levels. |
Chronic is the extended or long-term exposure to a stressor, conventionally taken to include at least a tenth of the life-span of a species. |
Concentrationresponse curve is a curve describing the relationship between response in the test population and exposure concentration. |
Contaminant is any chemical existing in the environment above background levels and representing, or potentially representing, an adverse health or environmental risk. |
Contamination means the condition of land or water where any chemical substance or waste has been added at above background level and represents, or potentially represents, an adverse health or environmental impact. |
Control is treatment in a trial that duplicates all the conditions of the exposure treatments but contains no test material. |
Default conversion factors are numerical values that are used to convert a measure of toxicity to another measure of toxicity (for example, EC50 to a NOEC) when no experimentally determined values are available. |
Ecological investigation level (EIL) is the concentration of a contaminant above which further appropriate investigation and evaluation of the impact on ecological values will be required. The EILs are calculated using EC30 or lowest observed effect concentrations (LOEC) toxicity data. EILs are the sum of the added contaminant limit (ACL) and the ambient background concentration (ABC) and the limit is expressed in terms of total concentration. All EILs, whether generic, soil-specific or site-specific, only apply to soil to a depth of two metres below the current soil surface. |
ECx means effective concentration; the concentration which affects X% of a test population after a specified exposure time. |
End point assessment is a quantitative or quantifiable expression of the environmental value considered to be at risk in a risk analysis. |
Environmental fate means the destiny of a chemical or biological pollutant after its release into the natural environment. |
Environmental quality guideline is a generic term that applies to any guidelines that control the concentration of contaminants in various environmental compartments (for example, water, sediment, soil). |
Freundlich adsorption isotherm is an empirical equation that describes the adsorption of a contaminant to soil. The equation for this is x/m = KfCel/n, where x/m is the concentration of the contaminant in soil (mg/kg), Ce is the contaminant concentration in the aqueous phase at equilibrium (mg/L), Kf is the equilibrium constant (the Freundlich adsorption constant) and l/n is the contaminant-specific exponent. |
Generic soil quality guidelines describe a single concentration-based value that applies to all Australian soils that have a particular land use. These are derived when normalisation relationships are not available. Compare these with soil-specific soil quality guidelines. |
Indicator means a biotic characteristic of the environment, for example, a plant end point that provides evidence of the occurrence or magnitude of exposure or effects. |
Kd (see water to soil partition coefficient). |
Koc (see organic carbonwater partition coefficient) |
Kow (see octanolwater partition coefficient) |
Leaching involves the dissolving of contaminants in soil and subsequent downward transport to groundwater or surface water bodies. |
Leachate is water that has percolated through a column of soil. |
LOEC is the lowest observed effect concentration (level); the lowest concentration of a material used in a test that has a statistically significant effect on the exposed population of test organisms compared to the control. |
Logistic curve is a function fitting the general equation y = k/ (1+ea+bt) where t represents time, y the body weight or population size, a and b are model-specific parameters. This mathematical function with parameters can be adjusted so that the function closely describes a set of empirical data. Statistical models are curve-fitted to data where the mathematical function used is selected for its numerical properties. |
NOEC means no observed effect concentration; the highest concentration of a test substance to which organisms are exposed that does not cause any observed and statistically significant adverse effects on the organisms compared to the controls. |
Normalisation relationships are empirical, generally linear, relationships that can predict the toxicity of a contaminant to an organism using soil physicochemical properties. These are used in the EIL derivation methodology to generate soil-specific soil quality guidelines. |
Octanolwater partitioning (Kow) means the ratio of a chemical’s solubility in n-octanol and water at equilibrium. This is widely used as a surrogate for the ability of a contaminant to accumulate in organisms and to biomagnify. These are often expressed in the logarithmic form (that is, log Kow). Chemicals with a log Kow value ≥4 are considered in this guideline to have the potential to biomagnify. There is a linear relationship between log Kow and log Koc values. Thus, Kow can also be used to indicate the ability of chemical to leach to groundwater. A log Kow value <2 indicates a chemical has the potential to leach to groundwater. |
Organic carbonwater partition coefficient (Koc) means the ratio of a chemical’s solubility in organic carbon and water at equilibrium. This is widely used as a surrogate for the ability of a contaminant to accumulate in soils and conversely to leach to groundwater or to be removed by surface run-off. These are often expressed in the logarithmic form (that is, log Koc). Chemicals with a log Koc <2.4 were considered, in this guideline, to be mobile and therefore have the ability in some soils to leach to groundwater. |
Precautionary principle is the general principle by which all that can reasonably be expected is done to prevent unnecessary risks. |
Reference site is a relatively uncontaminated site used for comparison with contaminated sites in environmental monitoring studies or used for the assessment of ambient background concentrations of contaminants. |
Risk assessment is a process intended to calculate or estimate the risk to a given target organism, system or sub-population, including the identification of attendant uncertainties, following exposure to a particular agent, taking into account the inherent characterisations of the agent of concern as well as the characterisation of the specific target. |
Risk means the probability in a certain timeframe that an adverse outcome will occur in a person, a group of people, plants, animals and/or the ecology of a specified area that is exposed to a particular dose or concentration of a chemical substance; that is, it depends on both the level of toxicity of the chemical substance and the level of exposure to it. |
Secondary poisoning is the product of biomagnification and toxicity. |
Soil quality guideline (SQG) is a collective term used to describe any quantitative or qualitative limit that controls the concentration of contaminants in soils. Ecological investigation levels (EILs) are a type of SQG. |
Soil-specific soil quality guidelines is a suite of concentration-based values, where each value applies to a soil with different physicochemical properties. These values take into account properties of soils that modify the bioavailability and toxicity of contaminants. These can only be derived if normalisation relationships are available. Compare these to generic SQGs. |
Speciation is the exact chemical form of contaminant in which an element occurs in a sample. |
Species sensitivity distribution (SSD) is a suite of methods that are the main method used to derive quality guidelines for contaminants in different compartments of the environment (for example, soil, water, sediment). Basically, these plot toxicity data (one value per species) as a cumulative frequency distribution against the concentration at which the toxic effect occurs. A statistical distribution is then fitted to the plot from which it can be estimated what concentration is required to protect any chosen percentage of species. In Australia, the SSD method used to derive guidelines uses the Burr type III family of distributions and is called the BurrliOZ method. |
Statistically significant effects are effects (responses) in the exposed population which are different from those in the controls at a statistical probability level of p <0.05. |
Steady state is the non-equilibrium state of a system in which matter flows in and out at equal rates so that all of the components remain at constant concentrations (dynamic equilibrium). |
Water to soil partition coefficient (Kd) is the ratio of the concentration of a contaminant in soil pore water to that in the solid phase of soil at equilibrium. The units are L/kg. This contaminant property is affected by physicochemical properties of the contaminant and the soil. This property is usually expressed as a logarithm (that is, log Kd). In this guideline, chemicals with log Kd <3 are considered to have the potential to leach. |
ABC | ambient background concentration |
ACL | added contaminant limit |
ACR | acute-to-chronic ratio |
AF | assessment factor |
ALF | ageing/leaching factor |
ANZECC | Australia and New Zealand Environment and Conservation Council |
ARMCANZ | Agriculture and Resource Management Council of Australia and New Zealand |
BAF | bioaccumulation factor |
BCF | bioconcentration factor |
BMF | biomagnification factor |
CCME | Canadian Council of Ministers of the Environment |
CEC | cation exchange capacity |
DAF | dilution and attenuation factor |
DOC | dissolved organic carbon |
DTA | direct toxicity assessment |
Eco-SSL | ecological soil screening level |
ECB | European Chemicals Bureau |
EC30 | 30% effect concentration |
EGV | environmental guideline value |
EIL | ecological investigation level |
ERA | ecological risk assessment |
EqP | equilibrium partitioning method |
EQG | environmental quality guideline |
EU | European Union |
HC | hazardous concentration |
HIL | health investigation level |
LOEC | lowest observed effect concentration |
MATC | maximum acceptable toxicant concentration |
NBRP | National Biosolids Research Program |
NEPC | National Environment Protection Council |
NEPM | National Environment Protection Measure |
NHMRC | National Health and Medical Research Council |
NOEC | no observed effect concentration |
OECD | Organisation for Economic Cooperation and Development |
OM | organic matter |
PC | protective concentration |
PLS | partial least squares |
PNEC | predicted no-effect concentration |
QAAR | quantitative activityactivity relationship |
QSAR | quantitative structureactivity relationship |
QSPR | quantitative structureproperty relationship |
SGV | soil guideline value |
SIN | substrate-induced nitrification |
SQG | soil quality guideline |
SQV | soil quality value |
SSD | species sensitivity distribution |
US EPA | United States Environmental Protection Agency |
TGD | technical guidance document |
TRV | toxicity reference value |
TV | trigger value |
USA | United States of America |
VROM | Ministry of Housing, Spatial Planning, and the Environment (Netherlands) |
WHC | water holding capacity |
WQG | water quality guideline |
[1] In cases where the LOEC and/or EC30 data are not available but other measures of toxicity are then the latter will be divided by conversion factors to obtain the former (refer to Glossary, Section 2.4.2.1 and Table 8 for further details).
[2] Protection is provided in terms of the percentage of species and soil microbial processes because the method used to derive EILs is a species sensitivity distribution method.
[3] This occurs because as exposure to a toxicant increases, the external ambient concentration needed to cause a toxic effect decreases.
[4] The H can also be expressed on a mass basis (e.g. mg/kg).
[5] The Australasian Ecotoxicology Database is in the process of being placed on the CSIRO website. It should be available in 2011.
[6] This is done by comparing values predicted by the non-Australian normalisation relationships to Australian toxicity data.
[7] The geometric mean is analogous to the normal arithmetic mean except that the values are logged before summing and being divided by the number of data points. The value is then anti-logged to provide the geometric mean. The formula for this is
Geometric mean = anti-log [(logA + log B +…..logN)/n] (equation 13)
In determining the geometric mean the data can be logged to any base (e.g. log10, log2 or the natural log) as long as the same base is used throughout equation 13.
The reason for using the geometric mean rather than the arithmetic mean is that the geometric mean is not affected as much by extremely low or high values. For example, the geometric and arithmetic means of a data set consisting of 10, 25, 40 are 21.5 and 25 respectively. If a value of 400 was added to the same data set then the geometric and arithmetic means would be 45 and 119 respectively.