Schedule 1—Pathology services table
Note: See section 5.
Part 1—Preliminary
Division 1.1—Interpretation
1.1.1 Abbreviations
(1) The abbreviations in Part 3 may be used to identify particular pathology services or groups of pathology services.
(2) The names of services or drugs not listed in Part 3 must be written in full.
Division 1.2—General application provisions
1.2.1 Precedence of items
(1) If a service is described:
(a) in an item in general terms; and
(b) in another item in specific terms;
only the item that describes the service in specific terms applies to the service.
(2) Subject to subclause (3), if:
(a) subclause (1) does not apply; and
(b) a service is described in 2 or more items;
only the item that provides the lower or lowest fee for the service applies to the service.
(3) If an item is expressed to include a pathology service that is described in another item, the other item does not apply to the service in addition to the first‑mentioned item, whether or not the services described in the 2 items are requested separately.
1.2.2 Circumstances in which services rendered following 2 requests to be taken to have been rendered following one request
(1) In subclause (2):
service includes assay, estimation and test.
(2) Two or more pathology services (other than services to which, under clause 1.2.3, 2.1.1 or 2.2.2, this clause does not apply) rendered for a patient following 2 or more requests are taken to have been rendered following a single request if:
(a) the services are listed in the same item; and
(b) that item is not item 74990 or 74991; and
(c) the patient’s need for the services was determined under subsection 16A(1) of the Act on the same day even if the services are rendered by an approved pathology practitioner on more than 1 day.
1.2.3 Services to which clause 1.2.2 does not apply
(1) Clause 1.2.2 does not apply to a pathology service described in subclause (2) if:
(a) under a request for a service, other than a request for a service described in paragraph (2)(a), no more than 6 tests are requested; and
(b) the tests are performed within 6 months of the request; and
(c) the pathology provider of the service writes on the account for the service that the service has a rule 3 exemption.
(2) For subclause (1), the pathology services are:
(a) estimation of prothrombin time (INR) for a patient undergoing anticoagulant therapy; and
(b) quantitative estimation of lithium for a patient undergoing lithium therapy; and
(c) a service described in item 65070 in relation to a patient undergoing chemotherapy for neoplastic disease or immunosuppressant therapy; and
(d) a service described in item 65070 in relation to
clozaril, ticlopidine hydrochloride, methotrexate, gold, sulphasalazine or penicillamine therapy of a patient; and
(e) a service described in any of items 66500 to 66512, in relation to methotrexate or leflunomide therapy of a patient; and
(f) quantitative estimation of urea, creatinine and electrolytes in relation to:
(i) cis‑platinum or cyclosporin therapy of a patient; or
(ii) chronic renal failure of a patient being treated
in a dialysis program conducted by a recognised hospital; and
(g) quantitative estimation of albumin and calcium in relation to therapy of a patient with vitamin D, its metabolites or analogues; and
(h) quantitative estimation of calcium, phosphate, magnesium, urea, creatinine and electrolytes for a cancer patient receiving bisphosphonate infusions.
1.2.4 Referral of designated tests by one pathology practitioner to another
(1) In this clause:
designated test means a pathology test relating to a patient episode that is a test of a kind mentioned in item 65150, 65175, 66650, 66695, 66711, 66722, 66785, 66800, 66812, 66819, 66825, 69384, 69494, 71089, 71153 or 77165.
(2) This clause applies if one or more designated tests are referred by a referring APP to a receiving APP in another approved pathology authority.
(3) If a referring APP has rendered one or more designated tests:
(a) the amount mentioned in item 65150, 65153, 65175, 65176, 65177, 65178, 66650, 66695, 66698, 66701, 66704, 66707, 66711, 66722, 66725, 66728, 66731, 66785, 66800, 66803, 66812, 66819, 66825, 69384, 69387, 69390, 69393, 69396, 69494, 69495, 71089, 71091, 71153, 71155, 71157, 77165, 71166 or 71167 (as the case may be) is payable for each designated test rendered by the referring APP; and
(b) subject to subclause (5), the amount mentioned in item 65158, 65181, 66652, 66697, 66715, 66724, 66790, 66805, 66817, 66821, 66827, 69401, 69498, 71092, 71156 or 71170 (as the case may be) is payable for each designated test rendered by the receiving APP.
(4) If a referring APP has not rendered a designated test:
(a) for the first designated test that is rendered by the receiving APP—the amount mentioned in item 65157, 65180, 66651, 66696, 66714, 66723, 66789, 66804, 66816, 66820, 66826, 69400, 69497, 71090, 71154 or 71169 (as the case may be) is payable; and
(b) for each subsequent designated test (if any) that is rendered by the receiving APP—subject to subclause (6), the amount mentioned in item 65158, 65181, 66652, 66697, 66715, 66724, 66790, 66805, 66817, 66821, 66827, 69401, 69498, 71092, 71156 or 71170 (as the case may be) is payable for each test rendered.
(5) For paragraph (3)(b), the maximum number of designated tests for which the fee mentioned in the relevant item is payable is as follows:
(a) for item 66652, 66715, 66790, 66817, 66821 or 66827:
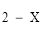
(b) for item 65158, 66805, 69498 or 71092:

(c) for item 71156 or 71170:
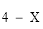
(d) for item 65181 or 66724:

where:
X is the number of designated tests rendered by a referring APP.
(6) For paragraph (4)(b), the maximum number of designated tests for which the fee mentioned in the relevant item is payable is as follows:
(a) for item 66652, 66715, 66790, 66817, 66821 or 66827—1;
(b) for item 65158, 66805, 69498 or 71092—2;
(c) for item 71156 or 71170—3;
(d) for item 65181 or 66724—4.
(7) Items in Group P10 (Patient episode initiation) do not apply to a receiving APP in subclause (2).
1.2.5 Items not to be split
Except as stated in clause 1.2.4 the amount mentioned in an item is payable only to one approved pathology practitioner for a single patient episode.
1.2.6 Certain pathology services to be treated as one service
(1) In this clause:
general practitioner means a medical practitioner who:
(a) is not a consultant physician in any specialty; and
(b) is not a specialist in any specialty.
set of pathology services has the meaning given by subclause 1.2.7(1).
(2) If a general practitioner, participating midwife or participating nurse practitioner requests a set of pathology services, the pathology services in the set are to be treated as individual pathology services in accordance with this clause.
(3) If the fee mentioned in an item that describes any of the services in the set of pathology services is higher than the fees mentioned in the other items that describe the services in the set:
(a) the pathology service described in the first‑mentioned item is to be treated as one pathology service; and
(b) either:
(i) the pathology service in the set that is described in the item that mentions the second‑highest fee is to be treated as one pathology service; or
(ii) if 2 or more items that describe any of those services mentions the second‑highest fee—the pathology service described in the item that mentions the second‑highest fee, and has the lowest item number, is to be treated as one pathology service; and
(c) the pathology services in the set, other than the services that are to be treated as one pathology service under paragraphs (a) and (b), are to be treated as one pathology service.
(4) If the fees mentioned in 2 or more items that describe any of the services in the set of pathology services are the same, and higher than the fees mentioned in the other items that describe the services in the set:
(a) the pathology service in the set that is described in the item that mentions the highest fee, and has the lowest item number, is to be treated as one pathology service; and
(b) the pathology service in the set that is described in the item that mentions the highest fee, and has the second‑lowest item number, is to be treated as one pathology service; and
(c) the pathology services in the set, other than the services that are to be treated as one pathology service under paragraphs (a) and (b), are to be treated as one pathology service.
(5) If pathology services are to be treated as one pathology service under paragraph (3)(c) or (4)(c), the fee for the one pathology service is the highest fee mentioned in any of the items that describe the pathology services that are to be treated as the one pathology service.
1.2.7 Meaning of set of pathology services
(1) In clause 1.2.6:
set of pathology services means a group of pathology services:
(a) that consist of services that are described in at least 4 different items; and
(b) all of which are requested in a single patient episode; and
(c) each of which relates to a patient who is not an admitted patient of a hospital; and
(d) none of which is referred to in item 66900, 69484, 73053 or 73055.
(2) However, a set of pathology services does not include the following items:
(a) an item in Group P10 (Patient episode initiation), Group P11 (Specimen referred), Group P12 (Management of bulk‑billed services) or Group P13 (Bulk billing incentive for episodes consisting of a P10 service);
(b) if a service is requested by an approved pathology practitioner of an approved pathology authority and rendered by another approved pathology practitioner of an approved pathology authority that is not related to the approved pathology authority of the first‑mentioned approved pathology practitioner—item 65079, 65082, 65157, 65158, 65166, 65180, 65181, 66606, 66610, 66639, 66642, 66651, 66652, 66663, 66666, 66696, 66697, 66714, 66715, 66723, 66724, 66780, 66783, 66789, 66790, 66792, 66804, 66805, 66816, 66817, 66820, 66821, 66826, 66827, 66832, 66834, 66837, 69325, 69328, 69331, 69379, 69383, 69400, 69401, 69419, 69451, 69489, 69492, 69497, 69498, 69500, 71076, 71090, 71092, 71096, 71148, 71154, 71156, 71169, 71170, 73309, 73312, 73315, 73318, 73321 or 73324.
(3) An approved pathology authority is related to another approved pathology authority for paragraph (2)(b) if:
(a) both approved pathology authorities are employed (including employed under contract) by the same person, whether or not the person is also an approved pathology authority; or
(b) either of the approved pathology authorities is employed (including employed under contract) by the other; or
(c) both approved pathology authorities are corporations and are connected entities within the meaning of the Corporations Act 2001; or
(d) the approved pathology authorities are partners (whether or not either or both of the approved pathology authorities are individuals and whether or not other persons are in partnership with either or both of the approved pathology authorities); or
(e) both approved pathology authorities are operated by the Commonwealth or an authority of the Commonwealth; or
(f) both approved pathology authorities are operated by the same State or internal Territory or an authority of the same State or internal Territory.
1.2.8 Satisfying requirements described in pathology service
Unless the contrary intention appears, a requirement contained in the description of a pathology service in Part 2 is satisfied if:
(a) for a requirement for information—the information:
(i) is included in the request for the service; or
(ii) was supplied in writing on an earlier occasion to
the approved pathology authority that rendered
the service, and has been kept by the approved pathology authority; or
(b) for a requirement for laboratory test results—the results are:
(i) included in the request for the service; or
(ii) obtained from another laboratory test performed in the same patient episode; or
(iii) included in results from an earlier laboratory test that have been kept by the approved pathology authority.
1.2.9 Application of items—services provided with autologous injections of blood or blood products
An item in the table does not apply to a service mentioned in the item if the service is provided to a patient at the same time as, or in connection with, an injection of blood or a blood product that is autologous.
Part 2—Services and fees
Division 2.1—Group P1: haematology
2.1.1 Services to which clause 1.2.2 does not apply
(1) Clause 1.2.2 does not apply to a pathology service described in item 65060, 65070, 65120, 65123, 65126, 65129, 65150, 65153 or 65156 if:
(a) the service is rendered in relation to one or more specimens taken on any of not more than 6 occasions in a period of 24 hours; and
(b) the service is rendered to an inpatient of a hospital; and
(c) the service is rendered in relation to each specimen as soon as possible after the specimen is taken; and
(d) the pathology provider of the service writes on the account for the service that the service has a rule 3 exemption.
(2) Clause 1.2.2 does not apply to a pathology service described in item 65109 or 65110 if:
(a) the service is rendered:
(i) for a service described in item 65109—on one of not more than 5 occasions in a period of 24 hours; and
(ii) for a service described in item 65110—on one of not more than 2 occasions in a period of 24 hours; and
(b) the service was requested on a separate occasion to any other occasions on which the service was requested in the period of 24 hours; and
(c) the pathology provider of the service writes on the account for the service that the service has a rule 3 exemption.
2.1.2 Item taken to refer only to the first service of a particular kind
(1) For an item in Group P1 (Haematology):
(a) if pathology services of a kind mentioned in item 65090 or 65093 are rendered for a patient during a period when the patient is in hospital, the item applies only to the first pathology service of that kind rendered for the patient during the period; and
(b) if:
(i) tests (except tests mentioned in item 65099, 65102, 65105 or 65108) are carried out in relation to a patient episode; and
(ii) specimen material from the patient episode is stored; and
(iii) in response to a request made within 14 days of the patient episode, further tests (except tests mentioned in item 65099, 65102, 65105 or 65108) are carried out on the stored material;
the later tests and the earlier tests are taken to be part of one patient episode.
(2) Items 65102 and 65108 apply only if a minimum of 6 units are issued for a patient’s care in any 1 day.
(3) For items 65099 and 65102:
compatibility tests by crossmatch means that, in addition to all the tests mentioned in paragraphs (a) and (b) of the item, donor red cells from each unit must have been tested directly against the serum of the patient by one or more accepted crossmatching techniques.
Group P1—Haematology |
Item | Pathology service | Fee ($) |
65060 | Haemoglobin, erythrocyte sedimentation rate, blood viscosity—one or more tests | 7.85 |
65066 | Examination of: (a) a blood film by special stains to demonstrate Heinz bodies, parasites or iron; or (b) a blood film by enzyme cytochemistry for neutrophil alkaline phosphatase, alpha‑naphthyl acetate esterase or chloroacetate esterase; or | 10.40 |
| (c) a blood film using any other special staining methods including periodic acid Schiff and Sudan Black; or (d) a urinary sediment for haemosiderin; including a service described in item 65072 | |
65070 | Erythrocyte count, haematocrit, haemoglobin, calculation or measurement of red cell index or indices, platelet count, leucocyte count and manual or instrument generated differential count (not being a service where haemoglobin only is requested)—one or more instrument‑generated set of results from a single sample and (if performed): (a) a morphological assessment of a blood film; and (b) any service in item 65060 or 65072 | 16.95 |
65072 | Examination for reticulocytes including a reticulocyte count by any method—one or more tests | 10.20 |
65075 | Haemolysis or metabolic enzymes—assessment by one or more of the following tests: (a) erythrocyte autohaemolysis test; (b) erythrocyte osmotic fragility test; (c) sugar water test; (d) G–6–PD (qualitative or quantitative) test; (e) pyruvate kinase (qualitative or quantitative) test; (f) acid haemolysis test; (g) quantitation of muramidase in serum or urine; (h) Donath Landsteiner antibody test; (i) other erythrocyte metabolic enzyme tests | 51.95 |
65078 | Tests for the diagnosis of thalassaemia consisting of haemoglobin electrophoresis or chromatography and at least 2 of: (a) examination for HbH; or (b) quantitation of HbA2; or (c) quantitation of HbF; including (if performed) any service described in item 65060 or 65070 | 90.20 |
65079 | A test described in item 65078 if rendered by a receiving APP—one or more tests | 90.20 |
65081 | Tests for the investigation of haemoglobinopathy consisting of haemoglobin electrophoresis or chromatography and at least one of: (a) heat denaturation test; or (b) isopropanol precipitation test; or (c) tests for the presence of haemoglobin S; or (d) quantitation of any haemoglobin fraction (including S, C, D, E); including (if performed) any service described in item 65060, 65070 or 65078 | 96.60 |
65082 | A test described in item 65081 if rendered by a receiving APP—one or more tests | 96.60 |
65084 | Bone marrow trephine biopsy—histopathological examination of sections of bone marrow and examination of aspirated material (including clot sections where necessary), including (if performed) any test described in item 65060, 65066 or 65070 | 165.85 |
65087 | Bone marrow—examination of aspirated material (including clot sections where necessary), including (if performed) any test described in item 65060, 65066 or 65070 | 83.10 |
65090 | Blood grouping (including back‑grouping if performed)—ABO and Rh (D antigen) | 11.15 |
65093 | Blood grouping—Rh phenotypes, Kell system, Duffy system, M and N factors or any other blood group system—one or more systems, including item 65090 (if performed) | 22.00 |
65096 | Blood grouping (including back‑grouping if performed), and examination of serum for Rh and other blood group antibodies, including: (a) identification and quantitation of any antibodies detected; and (b) (if performed) any test described in item 65060 or 65070 | 41.00 |
65099 | Compatibility tests by crossmatch—all tests performed on any 1 day for up to 6 units, including: (a) all grouping checks of the patient and donor; and (b) examination for antibodies and, if necessary, identification of any antibodies detected; and (c) (if performed) any tests described in item 65060, 65070, 65090 or 65096 | 108.90 |
65102 | Compatibility tests by crossmatch—all tests performed on any 1 day in excess of 6 units, including: (a) all grouping checks of the patient and donor; and (b) examination for antibodies and, if necessary, identification of any antibodies detected; and (c) (if performed) any tests described in item 65060, 65070, 65090, 65096, 65099 or 65105 | 164.60 |
65105 | Compatibility testing using at least a 3 cell panel and issue of red cells for transfusion—all tests performed on any 1 day for up to 6 units, including: (a) all grouping checks of the patient and donor; and (b) examination for antibodies and, if necessary, identification of any antibodies detected; and (c) (if performed) any tests described in item 65060, 65070, 65090 or 65096 | 108.90 |
65108 | Compatibility testing using at least a 3 cell panel and issue of red cells for transfusion—all tests performed on any 1 day in excess of 6 units, including: (a) all grouping checks of the patient and donor; and (b) examination for antibodies and, if necessary, identification of any antibodies detected; and (c) (if performed) any tests described in item 65060, 65070, 65090, 65096, 65099 or 65105 | 164.60 |
65109 | Release of fresh frozen plasma or cryoprecipitate for the use in a patient for the correction of a coagulopathy—one release | 12.90 |
65110 | Release of compatible fresh platelets for the use in a patient for platelet support as prophylaxis to minimise bleeding or during active bleeding—one release | 12.90 |
65111 | Examination of serum for blood group antibodies (including identification and, if necessary, quantitation of any antibodies detected) | 23.20 |
65114 | One or more of the following tests: (a) direct Coombs (antiglobulin) test; (b) qualitative or quantitative test for cold agglutinins or heterophil antibodies | 9.10 |
65117 | One or more of the following tests: (a) spectroscopic examination of blood for chemically altered haemoglobins; (b) detection of methaemalbumin (Schumm’s test) | 20.25 |
65120 | Prothrombin time (including INR where appropriate), activated partial thromboplastin time, thrombin time (including test for the presence of heparin), test for factor XIII deficiency (qualitative), Echis test, Stypven test, reptilase time, fibrinogen, or one of fibrinogen degradation products, fibrin monomer or D‑dimer—one test | 13.70 |
65123 | Two tests described in item 65120 | 20.35 |
65126 | Three tests described in item 65120 | 27.85 |
65129 | Four or more tests described in item 65120 | 35.50 |
65137 | A test for the presence of lupus anticoagulant, not being a service connected with a service to which item 65175, 65176, 65177, 65178 or 65179 applies | 25.35 |
65142 | Confirmation or clarification of an abnormal or indeterminate result of a test mentioned in item 65175, by testing a specimen collected on a different day—one or more tests | 25.35 |
65144 | Platelet aggregation in response to ADP, collagen, 5HT, ristocetin or other substances, or heparin, low molecular weight heparins, heparinoid or other drugs—one or more tests | 56.55 |
65147 | Quantitation of anti‑Xa activity when monitoring is required for a patient receiving a low molecular weight heparin or heparinoid—one test | 37.90 |
65150 | Quantitation of von Willebrand factor antigen, von Willebrand factor activity (ristocetin cofactor assay), von Willebrand factor collagen binding activity, factor II, factor V, factor VII, factor VIII, factor IX, factor X, factor XI, factor XII, factor XIII, Fletcher factor, Fitzgerald factor, circulating coagulation factor inhibitors other than by Bethesda assay—one test | 70.90 |
65153 | Two tests described in item 65150 | 141.85 |
65156 | Three or more tests described in item 65150 | 212.75 |
65157 | A test described in item 65150, if rendered by a receiving APP, where no tests in the item have been rendered by the referring APP—one test | 70.90 |
65158 | A test described in item 65150, if rendered by a receiving APP, where one or more tests in the item have been rendered by the referring APP—one test | 70.90 |
65159 | Quantitation of circulating coagulation factor inhibitors by Bethesda assay—one test | 70.90 |
65162 | Examination of a maternal blood film for the presence of fetal red blood cells (Kleihauer test) | 10.45 |
65165 | Detection and quantitation of fetal red blood cells in the maternal circulation by detection of red cell antigens using flow cytometric methods including (if performed) any test described in item 65070 or 65162 | 34.45 |
65166 | A test described in item 65165 if rendered by a receiving APP—one or more tests | 34.45 |
65171 | A test for the presence of antithrombin III deficiency, protein C deficiency, protein S deficiency or activated protein C resistance in a first degree relative of a person who has a proven deficiency mentioned in this item—one or more tests | 25.35 |
65175 | A test for the presence of antithrombin III deficiency, protein C deficiency, protein S deficiency, lupus anticoagulant, activated protein C resistance, if the request for the test specifically identifies that the patient has a history of venous thromboembolism—quantitation by one or more techniques—one test | 25.35 |
65176 | Two tests described in item 65175 | 48.65 |
65177 | Three tests described in item 65175 | 71.95 |
65178 | Four tests described in item 65175 | 95.20 |
65179 | Five tests described in item 65175 | 118.50 |
65180 | A test described in item 65175, if rendered by a receiving APP, where no tests in the item have been rendered by the referring APP—one test | 25.35 |
65181 | A test described in item 65175, if rendered by a receiving APP, where one or more tests in the item have been rendered by the referring APP—one test | 23.30 |
Division 2.2—Group P2: chemical
2.2.1 Creatinine ratios
A pathology service mentioned in an item in Group P2 (Chemical) (except item 66500) that:
(a) involves the measurement of a substance in urine; and
(b) requires calculation of a substance/creatinine ratio;
is taken to include the measurement of creatinine necessary for the calculation.
2.2.2 Services to which clause 1.2.2 does not apply
Clause 1.2.2 does not apply to a pathology service described in item 66500, 66503, 66506, 66509, 66512, 66584 or 66800 if:
(a) the service is rendered in relation to one or more specimens taken on any of not more than 6 occasions in a period of 24 hours; and
(b) the service is rendered to an inpatient of a hospital; and
(c) the service is rendered in relation to each specimen as soon as possible after the specimen is taken; and
(d) the pathology provider of the service writes on the account for the service that the service has a rule 3 exemption.
2.2.3 Limitation on certain items
(1) For any particular patient, the following items are applicable not more than twice in a 12 month period:
(a) items 66539 and 66607;
(b) item 66605, 66606 or 66610.
(2) For any particular patient, each of items 66551 and 66660 is applicable not more than 4 times in a 12 month period.
(3) For any particular patient, each of items 66554 and 66830 is applicable not more than 6 times in a 12 month period.
(4) For any particular patient, item 66626 is applicable not more than 36 times in a 12 month period.
(5) For any particular patient, item 66750 or 66751 is applicable not more than once in a pregnancy.
(6) For any particular patient, item 66517 is applicable not more than 3 times in a pregnancy.
(7) For any particular patient, item 66838 is applicable not more than once in a 12 month period.
2.2.5 Thyroid function testing
(1) For item 66719:
abnormal level of TSH means a level of TSH that is outside the normal reference range for the particular method of assay used to determine the level.
(2) Unless paragraph (a) of item 66719 is satisfied, the amount mentioned in the item is not payable for a pathology service mentioned in the item unless the pathologist who renders the service has a written statement from the medical practitioner who requested the service that satisfies subclause (3).
(3) The written statement from the medical practitioner must indicate:
(a) that the tests are required for a particular purpose, being a purpose mentioned in paragraph (b) of item 66719; or
(b) that the medical practitioner who requested the tests suspects the patient has pituitary dysfunction; or
(c) that the patient is on drugs that interfere with thyroid hormone metabolism or function.
2.2.6 Limitation on certain items
(1) For any particular patient, each of items 66655, 66659 and 66841 is applicable not more than once in a 12 month period.
(2) For any particular patient, each of items 66819, 66820, 66821, 66822, 66825, 66826, 66827 and 66828 is applicable not more than 3 times in a 6 month period.
2.2.7 Nutritional and toxicity metals testing
(1) In this clause:
metal toxicity testing group means items 66825, 66826, 66827, 66828, 66831 and 66832.
nutritional metals testing group means items 66819, 66820, 66821 and 66822.
(2) An item in the nutritional metals testing group or the metal toxicity testing group does not apply to a service performed if medicare benefits are paid or payable for tests that are performed for the same patient in 3 patient episodes requested within 6 months before the request for that service, under any of:
(a) that item; or
(b) the other item in the same group; or
(c) an item in the other group.
Group P2—Chemical |
Item | Pathology service | Fee ($) |
66500 | Quantitation in serum, plasma, urine or other body fluid (except amniotic fluid), by any method, except reagent tablet or reagent strip, (with or without reflectance meter) of acid phosphatase, alanine aminotransferase, albumin, alkaline phosphatase, ammonia, amylase, aspartate aminotransferase, bicarbonate, bilirubin (total), bilirubin (any fractions), C‑reactive protein, calcium (total or corrected for albumin), chloride, creatine kinase, creatinine, gamma glutamyl transferase, globulin, glucose, lactate dehydrogenase, lipase, magnesium, phosphate, potassium, sodium, total cholesterol, total protein, triglycerides, urate or urea—one test | 9.70 |
66503 | Two tests described in item 66500 | 11.65 |
66506 | Three tests described in item 66500 | 13.65 |
66509 | Four tests described in item 66500 | 15.65 |
66512 | Five or more tests described in item 66500 | 17.70 |
66517 | Quantitation of bile acids in blood in pregnancy | 19.65 |
66518 | Investigation of cardiac or skeletal muscle damage by quantitative measurement of creatine kinase isoenzymes, troponin or myoglobin in blood—tests performed on only one specimen in a 24 hour period | 20.05 |
66519 | Investigation of cardiac or skeletal muscle damage by quantitative measurement of creatine kinase isoenzymes, troponin or myoglobin in blood—tests performed on 2 or more specimens in a 24 hour period | 40.15 |
66536 | Quantitation of HDL cholesterol | 11.05 |
66539 | Electrophoresis of serum for demonstration of lipoprotein subclasses: (a) if the cholesterol is >6.5 mmol/L and triglyceride >4.0 mmol/L; or (b) in the diagnosis of types III and IV hyperlipidaemia | 30.60 |
66542 | Oral glucose tolerance test for the diagnosis of diabetes mellitus, that includes: (a) administration of glucose; and (b) at least 2 measurements of blood glucose; and (c) (if performed) any test described in item 66695 | 18.95 |
66545 | Oral glucose challenge test in pregnancy for the detection of gestational diabetes that includes: (a) administration of glucose; and (b) one or 2 measurements of blood glucose; and (c) (if performed) any test in item 66695 | 15.80 |
66548 | Oral glucose tolerance test in pregnancy for the diagnosis of gestational diabetes that includes: (a) administration of glucose; and (b) at least 3 measurements of blood glucose; and (c) (if performed) any test in item 66695 | 19.90 |
66551 | Quantitation of glycosylated haemoglobin performed in the management of established diabetes | 16.80 |
66554 | Quantitation of glycosylated haemoglobin performed in the management of pre‑existing diabetes where the patient is pregnant—including a service in item 66551 (if performed) | 16.80 |
66557 | Quantitation of fructosamine performed in the management of established diabetes—each test to a maximum of 4 tests in a 12 month period | 9.70 |
66560 | Microalbumin—quantitation in urine | 20.10 |
66563 | Osmolality, estimation by osmometer, in serum or in urine—one or more tests | 24.70 |
66566 | Quantitation of: (a) blood gases (including pO2, oxygen saturation and pCO2); and (b) bicarbonate and pH; including any other measurement (e.g. haemoglobin, lactate, potassium or ionised calcium) or calculation performed on the same specimen—one or more tests on one specimen | 33.70 |
66569 | Quantitation of blood gases, bicarbonate and pH as described in item 66566 on 2 specimens performed on any 1 day | 42.60 |
66572 | Quantitation of blood gases, bicarbonate and pH as described in item 66566 on 3 specimens performed on any 1 day | 51.55 |
66575 | Quantitation of blood gases, bicarbonate and pH as described in item 66566 on 4 specimens performed on any 1 day | 60.45 |
66578 | Quantitation of blood gases, bicarbonate and pH as described in item 66566 on 5 specimens performed on any 1 day | 69.35 |
66581 | Quantitation of blood gases, bicarbonate and pH as described in item 66566 on 6 or more specimens performed on any 1 day | 78.25 |
66584 | Quantitation of ionised calcium (except if performed as part of item 66566)—one test | 9.70 |
66587 | Urine acidification test for the diagnosis of renal tubular acidosis including the administration of an acid load, and pH measurements on 4 or more urine specimens and at least one blood specimen | 47.55 |
66590 | Calculus, analysis of one or more | 30.60 |
66593 | Ferritin—quantitation, except if requested as part of iron studies | 18.00 |
66596 | Iron studies, consisting of quantitation of: (a) serum iron; and (b) transferrin or iron binding capacity; and (c) ferritin | 32.55 |
66605 | Vitamins—quantitation of vitamin B1, B2, B3, B6 or C in blood, urine or other body fluid—one or more tests | 30.60 |
66606 | A test described in item 66605 if rendered by a receiving APP—one or more tests | 30.60 |
66607 | Vitamins—quantitation of vitamin A or E in blood, urine or other body fluid—one or more tests in a 6 month period | 75.75 |
66610 | A test described in item 66607 if rendered by a receiving APP—one or more tests | 75.75 |
66623 | All qualitative and quantitative tests on blood, urine or other body fluid for: (a) a drug or drugs of abuse (including illegal drugs and legally available drugs taken other than in appropriate dosage); or (b) ingested or absorbed toxic chemicals; including a service described in item 66800, 66803, 66806, 66812 or 66815 (if performed), but excluding: (c) the surveillance of sports people and athletes for performance improving substances; and (d) the monitoring of patients participating in a drug abuse treatment program | 41.50 |
66626 | Detection or quantitation or both of a drug, or drugs, of abuse or a therapeutic drug, on a sample collected from a patient participating in a drug abuse treatment program, including all tests on blood, urine or other body fluid, not including: (a) the surveillance of sports people and athletes for performance improving substances; and (b) the detection of nicotine and metabolites in smoking withdrawal programs | 24.10 |
66629 | Beta‑2‑microglobulin—quantitation in serum, urine or other body fluids—one or more tests | 20.10 |
66632 | Caeruloplasmin, haptoglobins, or prealbumin—quantitation in serum, urine or other body fluids—one or more tests | 20.10 |
66635 | Alpha‑1‑antitrypsin—quantitation in serum, urine or other body fluid—one or more tests | 20.10 |
66638 | Isoelectric focusing or similar methods for determination of alpha‑1‑antitrypsin phenotype in serum—one or more tests | 49.05 |
66639 | A test described in item 66638 if rendered by a receiving APP—one or more tests | 29.20 |
66641 | Electrophoresis of serum or other body fluid to demonstrate: (a) the isoenzymes of lactate dehydrogenase; or (b) the isoenzymes of alkaline phosphatase; including the preliminary quantitation of total relevant enzyme activity—one or more tests | 29.20 |
66642 | A test described in item 66641 if rendered by a receiving APP—one or more tests | 29.20 |
66644 | C‑1 esterase inhibitor—quantitation | 20.15 |
66647 | C‑1 esterase inhibitor—functional assay | 45.10 |
66650 | Alpha‑fetoprotein, CA‑15.3 antigen (CA15.3), CA‑19.9 antigen (CA19.9), CA‑125 antigen (C125), cancer associated serum antigen (CASA), carcinoembryonic antigen (CEA), human chorionic gonadotrophin (HCG), neuron specific enolase (NSE) thyroglobulin in serum or other body fluid, in the monitoring of malignancy or in the detection or monitoring of gestational trophoblastic disease or a hepatic or germ cell tumour—quantitation—one test | 24.35 |
66651 | A test described in item 66650, if rendered by a receiving APP, where no tests in the item have been rendered by the referring APP—one test | 24.35 |
66652 | A test described in item 66650, if rendered by a receiving APP, where one or more tests in the item have been rendered by the referring APP—one test | 20.30 |
66653 | Two or more tests described in item 66650 | 44.60 |
66655 | Prostate specific antigen—quantitation | 20.15 |
66656 | Prostate specific antigen (PSA) quantitation in the monitoring of previously diagnosed prostatic disease (including a test to which item 66655 applies) | 20.15 |
66659 | Prostate specific antigen (PSA), quantitation of 2 or more fractions of PSA and any derived index, including, if performed, a test described in item 66656, in the follow up of a PSA result that lies at or above the age‑related median but below the age‑related, method‑specific 97.5% reference limit | 37.30 |
66660 | Prostate specific antigen (PSA), quantitation of 2 or more fractions of PSA and any derived index, including, if performed, a test described in item 66656, in the follow up of a PSA result that lies at or above the age‑related, method‑specific 97.5% reference limit, but below 10 µg/L | 37.30 |
66662 | Quantitation of hormone receptors on proven primary breast or ovarian carcinoma or a metastasis from a breast or ovarian carcinoma or a subsequent lesion in the breast—one or more tests | 79.95 |
66663 | A test described in item 66662 if rendered by a receiving APP—one or more tests | 79.95 |
66665 | Lead quantitation in blood or urine (other than for occupational health screening purposes) to a maximum of 3 tests in a 6 month period—each test | 30.60 |
66666 | A test described in item 66665 if rendered by a receiving APP—one or more tests | 30.60 |
66667 | Quantitation of serum zinc in a patient receiving intravenous alimentation—each test | 30.60 |
66671 | Quantitation of serum aluminium in a patient in a renal dialysis program—each test | 36.90 |
66674 | Quantitation of: (a) faecal fat; or (b) breath hydrogen in response to loading with disaccharides; one or more tests within a 28 day period | 39.95 |
66677 | Test for tryptic activity in faeces in the investigation of diarrhoea of longer than 4 weeks duration in children under 6 years | 11.15 |
66680 | Quantitation of disaccharidases and other enzymes in intestinal tissue—one or more tests | 74.45 |
66683 | Enzymes—quantitation in solid tissue or tissues other than blood elements or intestinal tissue—one or more tests | 74.45 |
66686 | Performance of one or more of the following procedures: (a) growth hormone suppression by glucose loading; (b) growth hormone stimulation by exercise; (c) dexamethasone suppression test; (d) sweat collection by iontophoresis for chloride analysis; (e) pharmacological stimulation of growth hormone | 50.65 |
66695 | Quantitation in blood or urine of hormones and hormone binding proteins—ACTH, aldosterone, androstenedione, C‑peptide, calcitonin, cortisol, DHEAS, 11‑deoxycortisol, dihydrotestosterone, FSH, gastrin, glucagon, growth hormone, hydroxyprogesterone, insulin, LH, oestradiol, oestrone, progesterone, prolactin, PTH, renin, sex hormone binding globulin, somatomedin C(IGF –1), free or total testosterone, urine steroid fraction or fractions, vasoactive intestinal peptide—one test | 30.50 |
66696 | A test described in item 66695, if rendered by a receiving APP, where no tests in the item have been rendered by the referring APP | 30.50 |
66697 | A test described in item 66695, if rendered by a receiving APP, where one or more tests in the item have been rendered by the referring APP—each test to a maximum of 4 tests | 13.20 |
66698 | Two tests described in item 66695 | 43.70 |
66701 | Three tests described in item 66695 | 56.90 |
66704 | Four tests described in item 66695 | 70.15 |
66707 | Five or more tests described in item 66695 | 83.35 |
66711 | Quantitation in saliva of cortisol in: (a) the investigation of Cushing’s syndrome; or (b) the management of children with congenital adrenal hyperplasia; one test | 30.15 |
66712 | Two tests described in item 66711 | 43.05 |
66714 | A test described in item 66711, if rendered by a receiving APP, where no tests in the item have been rendered by the referring APP | 30.15 |
66715 | A test described in item 66711, if rendered by a receiving APP, where one test in the item has been rendered by the referring APP—one test | 12.85 |
66716 | TSH quantitation | 25.05 |
66719 | Thyroid function tests (comprising the service described in item 66716 and one or more of the following tests—free thyroxine, free T3, for a patient, if at least one of the following conditions is satisfied: (a) the patient has an abnormal level of TSH; (b) the tests are performed: (i) for the purpose of monitoring thyroid disease in the patient; or (ii) to investigate the sick euthyroid syndrome if the patient is an admitted patient; or (iii) to investigate dementia or psychiatric illness of the patient; or (iv) to investigate amenorrhoea or infertility of the patient; (c) the medical practitioner who requested the tests suspects the patient has a pituitary dysfunction; (d) the patient is on drugs that interfere with thyroid hormone metabolism or function | 34.80 |
66722 | TSH quantitation described in item 66716 and one test described in item 66695 | 37.90 |
66723 | A test described in item 66722, if rendered by a receiving APP, where no tests in the item have been rendered by the referring APP—one test | 37.90 |
66724 | A test described in item 66722, if rendered by a receiving APP, where one or more tests in the item have been rendered by the referring APP—one test | 13.15 |
66725 | TSH quantitation described in item 66716 and 2 tests described in item 66695 | 51.05 |
66728 | TSH quantitation described in item 66716 and 3 tests described in item 66695 | 64.20 |
66731 | TSH quantitation described in item 66716 and 4 tests described in item 66695 | 77.40 |
66734 | TSH quantitation described in item 66716 and 5 tests described in item 66695 | 90.55 |
66743 | Quantitation of alpha‑fetoprotein in serum or other body fluids during pregnancy except if requested as part of item 66750 or 66751 | 20.10 |
66749 | Amniotic fluid, spectrophotometric examination of, and quantitation of: (a) lecithin/sphingomyelin ratio; or (b) palmitic acid, phosphatidylglycerol or lamellar body phospholipid; or (c) bilirubin, including correction for haemoglobin; one or more tests | 32.95 |
66750 | Quantitation, in pregnancy, of any 2 of the following to detect foetal abnormality: (a) total human chorionic gonadotrophin (total HCG); (b) free alpha human chorionic gonadotrophin (free alpha HCG); (c) free beta human chorionic gonadotrophin (free beta HCG); (d) pregnancy associated plasma protein A (PAPP‑A); (e) unconjugated oestriol (uE3); (f) alpha‑fetoprotein (AFP); including (if performed) a service described in item 73527 or 73529 | 39.75 |
66751 | Quantitation, in pregnancy, of any 3 or more tests described in item 66750 | 55.25 |
66752 | Quantitation of acetoacetate, beta‑hydroxybutyrate, citrate, oxalate, total free fatty acids, cysteine, homocysteine, cystine, lactate, pyruvate or other amino acids and hydroxyproline (except if performed as part of item 66773 or 66776)—one test | 24.70 |
66755 | Two or more tests described in item 66752 | 38.85 |
66756 | Quantitation of 10 or more amino acids for the diagnosis of inborn errors of metabolism—up to 4 tests in a 12 month period on specimens of plasma, CSF and urine | 98.30 |
66757 | Quantitation of 10 or more amino acids for monitoring of previously diagnosed inborn errors of metabolism in one tissue type | 98.30 |
66758 | Quantitation of angiotensin converting enzyme, or cholinesterase—one or more tests | 24.70 |
66761 | Test for reducing substances in faeces by any method (except reagent strip or dipstick) | 13.15 |
66764 | Examination for faecal occult blood (including tests for haemoglobin and its derivatives in the faeces except by reagent strip or dip stick methods) with a maximum of 3 examinations on specimens collected on separate days in a 28 day period | 8.90 |
66767 | Two examinations described in item 66764 performed on separately collected and identified specimens | 17.85 |
66770 | Three examinations described in item 66764 performed on separately collected and identified specimens | 26.70 |
66773 | Quantitation of products of collagen breakdown or formation for the monitoring of patients with proven low bone mineral density and, if performed, a service described in item 66752—one or more tests | 24.65 |
66776 | Quantitation of products of collagen breakdown or formation for the monitoring of patients with metabolic bone disease or Paget’s disease of bone and, if performed, a service described in item 66752—one or more tests | 24.65 |
66779 | Adrenaline, noradrenaline, dopamine, histamine, hydroxyindoleacetic acid (5HIAA), hydroxymethoxymandelic acid (HMMA), homovanillic acid (HVA), metanephrines, methoxyhydroxyphenylethylene glycol (MHPG), phenylacetic acid (PAA) or serotonin—quantitation—one or more tests | 39.95 |
66780 | A test described in item 66779 if rendered by a receiving APP—one or more tests | 39.95 |
66782 | Porphyrins or porphyrins precursors—detection in plasma, red cells, urine or faeces—one or more tests | 13.15 |
66783 | A test described in item 66782 if rendered by a receiving APP—one or more tests | 13.15 |
66785 | Porphyrins or porphyrins precursors—quantitation in plasma, red cells, urine or faeces—one test | 39.95 |
66788 | Porphyrins or porphyrins precursors—quantitation in plasma, red cells, urine or faeces—2 or more tests | 65.85 |
66789 | A test described in item 66785 if rendered by a receiving APP, where no tests in the item have been rendered by the referring APP—one test | 39.95 |
66790 | A test described in item 66785, if rendered by a receiving APP, where one or more tests in the item have been rendered by the referring APP—one test | 25.90 |
66791 | Porphyrin biosynthetic enzymes—measurement of activity in blood cells or other tissues—one or more tests | 74.45 |
66792 | A test described in item 66791 if rendered by a receiving APP—one or more tests | 74.45 |
66800 | Quantitation in blood, urine or other body fluid by any method (except reagent tablet or reagent strip) of any of the following used therapeutically by the patient from whom the specimen was taken: amikacin, carbamazepine, digoxin, disopyramide, ethanol, ethosuximide, gentamicin, lignocaine, lithium, netilmicin, paracetamol, phenobarbitone, phenytoin, primidone, procainamide, quinidine, salicylate, theophylline, tobramycin, valproate or vancomycin—one test | 18.15 |
66803 | Two tests described in item 66800 | 30.50 |
66804 | A test described in item 66800 if rendered by a receiving APP, where no tests in the item have been rendered by the referring APP—one test | 18.15 |
66805 | A test described in item 66800, if rendered by a receiving APP, where one or more tests in the item have been rendered by the referring APP—one test | 12.35 |
66806 | Three tests described in item 66800 | 41.85 |
66812 | Quantitation, not elsewhere described in this table by any method or methods, in blood, urine or other body fluid, of a drug being used therapeutically by the patient from whom the specimen was taken—one test | 34.80 |
66815 | Two tests described in item 66812 | 59.55 |
66816 | A test described in item 66812 if rendered by a receiving APP, where no tests in the item have been rendered by the referring APP—one test | 34.80 |
66817 | A test described in item 66812, if rendered by a receiving APP, where one or more tests in the item have been rendered by the referring APP—one test | 24.75 |
66819 | Quantitation of copper, manganese, selenium or zinc (except if item 66667 applies), in blood, urine or other body fluid—one test | 30.60 |
66820 | A test described in item 66819 if rendered by a receiving APP, where no tests in the item have been rendered by the referring APP—one test | 30.60 |
66821 | A test described in item 66819, if rendered by a receiving APP, where one or more tests in the item have been rendered by the referring APP—one test | 21.80 |
66822 | Quantitation of copper, manganese, selenium or zinc (except if item 66667 applies), in blood, urine or other body fluid—2 or more tests | 52.45 |
66825 | Quantitation of aluminium (except if item 66671 applies), arsenic, beryllium, cadmium, chromium, gold, mercury, nickel or strontium, in blood, urine or other body fluid or tissue—one test | 30.60 |
66826 | A test described in item 66825 if rendered by a receiving APP where no tests have been rendered by the referring APP—one test | 30.60 |
66827 | A test described in item 66825, if rendered by a receiving APP, where one or more tests in the item have been rendered by the referring APP—one test | 21.80 |
66828 | Quantitation of aluminium (except if item 66671 applies), arsenic, beryllium, cadmium, chromium, gold, mercury, nickel or strontium, in blood, urine or other body fluid or tissue—2 or more tests | 52.45 |
66830 | Quantitation of BNP or NT‑proBNP for the diagnosis of heart failure in patients presenting with dyspnoea in a hospital emergency department | 58.50 |
66831 | Quantitation of copper or iron in liver tissue biopsy | 30.95 |
66832 | A test described in item 66831 if rendered by a receiving APP | 30.95 |
66833 | 25‑hydroxyvitamin D, quantification in serum, for the investigation of a patient who: (a) has signs or symptoms of osteoporosis or osteomalacia; or (b) has increased alkaline phosphatase and otherwise normal liver function tests; or (c) has hyperparathyroidism, hypo‑ or hypercalcaemia, or hypophosphataemia; or (d) is suffering from malabsorption (for example, because the patient has cystic fibrosis, short bowel syndrome, inflammatory bowel disease or untreated coeliac disease, or has had bariatric surgery); or (e) has deeply pigmented skin, or chronic and severe lack of sun exposure for cultural, medical, occupational or residential reasons; or (f) is taking medication known to decrease 25OH‑D levels (for example, anticonvulsants); or (g) has chronic renal failure or is a renal transplant recipient; or (h) is less than 16 years of age and has signs or symptoms of rickets; or (i) is an infant whose mother has established vitamin D deficiency; or (j) is an exclusively breastfed baby and has at least one other risk factor mentioned in a paragraph in this item; or (k) has a sibling who is less than 16 years of age and has vitamin D deficiency | 30.05 |
66834 | A test described in item 66833 if rendered by a receiving APP | 30.05 |
66835 | 1, 25‑dihydroxyvitamin D—quantification in serum, if the request for the test is made by, or on advice of, the specialist or consultant physician managing the treatment of the patient | 39.05 |
66836 | 1, 25‑dihydroxyvitamin D—quantification in serum, if: (a) a patient has hypercalcaemia; and (b) the request for the test is made by a general practitioner managing the treatment of the patient | 39.05 |
66837 | A test described in item 66835 or 66836 if rendered by a receiving APP | 39.05 |
66838 | Serum vitamin B12 test | 23.60 |
66839 | Quantification of vitamin B12 markers such as holoTranscobalamin or methylmalonic acid, where initial serum vitamin B12 result is low or equivocal | 42.95 |
66840 | Serum folate test and, if required, red cell folate test for a patient at risk of folate deficiency, including patients with malabsorption conditions, macrocytic anaemia or coeliac disease | 23.60 |
66841 | Quantitation of HbA1c (glycated haemoglobin) performed for the diagnosis of diabetes in asymptomatic patients at high risk | 16.80 |
66900 | Carbon‑labelled urea breath test using oral C‑13 or C‑14 urea, including the measurement of exhaled 13CO2 or 14CO2, (except if item 12533 of the general medical services table applies) for: (a) the confirmation of Helicobactor pylori colonisation; or (b) the monitoring of the success of eradication of Helicobactor pylori | 77.65 |
Division 2.3—Group P3: microbiology
2.3.1 Meaning of serial examinations or cultures
For an item in Group P3 (Microbiology):
serial examinations or cultures means:
(a) a series of examinations or cultures requested on one occasion whether or not:
(i) the materials are received on different days by the approved pathology practitioner; or
(ii) the examinations or cultures were requested on one or more request forms by the treating practitioner; and
(b) if:
(i) tests are carried out in relation to a patient episode; and
(ii) specimen material from the patient episode is stored; and
(iii) in response to a request made within 14 days of the patient episode, further tests are carried out on the stored material;
the later tests and the earlier tests are taken to be part of one patient episode.
2.3.2 Antigen detection
If a pathology service described in item 69316, 69317, 69319, 69494, 69495, 69496, 69497 or 69498 is rendered as a pathologist‑determinable service, the amount mentioned in the item is not payable for the service unless the recognised pathologist who renders the service records, in writing, the reasons for rendering the service.
2.3.3 Investigation for hepatitis serology
The fee applying in a single patient episode that includes any of items 69475, 69478 and 69481 is the fee specified for only one of those items.
2.3.4 Limitation on certain items
(1) For any particular patient, item 69336 is applicable not more than once in each period of 7 days.
(2) For any particular patient, the following items are applicable not more than once in a 12 month period:
(a) item 69482;
(b) item 69491 or 69492;
(c) item 69499 or 69500.
(3) For any particular patient, item 69418 or 69419 is applicable not more than twice in a 24 month period.
(4) For any particular patient, item 69380, 69488 or 69489 is applicable not more than twice in a 12 month period.
(5) For any particular patient, each of items 69445, 69451 and 69483 is applicable not more than 4 times in a 12 month period.
2.3.5 Hepatitis C viral RNA testing
For items 69499 and 69500:
Hepatitis C sero‑positive, for a patient, means 2 different assays of Hepatitis C antibodies are positive.
serological status is uncertain, for a patient, means any result where 2 different assays of Hepatitis C antibodies are inconclusive.
Group P3—Microbiology |
Item | Pathology service | Fee ($) |
69300 | Microscopy of wet film material other than blood, from one or more sites, obtained directly from a patient (not cultures) including (if performed): (a) differential cell count; or (b) examination for dermatophytes; or (c) dark ground illumination; or (d) stained preparation or preparations using any relevant stain or stains; one or more tests | 12.50 |
69303 | Culture and (if performed) microscopy to detect pathogenic micro‑organisms from nasal swabs, throat swabs, eye swabs and ear swabs (except swabs taken for epidemiological surveillance), including (if performed): (a) pathogen identification and antibiotic susceptibility testing; or (b) a service described in item 69300; specimens from one or more sites | 22.00 |
69306 | Microscopy and culture to detect pathogenic micro‑organisms from skin or other superficial sites, including (if performed): (a) pathogen identification and antibiotic susceptibility testing; or (b) a service described in items 69300, 69303, 69312 and 69318; one or more tests on one or more specimens | 33.75 |
69309 | Microscopy and culture to detect dermatophytes and other fungi causing cutaneous disease, from skin scrapings, skin biopsies, hair and nails (excluding swab specimens) and including (if performed): (a) the detection of antigens not elsewhere specified in this table; or (b) a service described in items 69300, 69303, 69306, 69312 and 69318; one or more tests on one or more specimens | 48.15 |
69312 | Microscopy and culture to detect pathogenic micro‑organisms from urethra, vagina, cervix or rectum (except for faecal pathogens), including (if performed): (a) pathogen identification and antibiotic susceptibility testing; or (b) a service described in items 69300, 69303, 69306 and 69318; one or more tests on one or more specimens | 33.75 |
69316 | Detection of Chlamydia trachomatis by any method—one test | 28.65 |
69317 | This item applies if: (a) one test described in item 69316 is performed; and (b) one test described in item 69494 is performed | 35.85 |
69318 | Microscopy and culture to detect pathogenic micro‑organisms from specimens of sputum (except when part of items 69324, 69327 and 69330), including (if performed): (a) pathogen identification and antibiotic susceptibility testing; or (b) a service described in items 69300, 69303, 69306 and 69312; one or more tests on one or more specimens | 33.75 |
69319 | This item applies if: (a) one test described in item 69316 is performed; and (b) 2 tests described in item 69494 are performed | 42.95 |
69321 | Microscopy and culture of post‑operative wounds, aspirates of body cavities, synovial fluid, CSF or operative or biopsy specimens, for the presence of pathogenic micro‑organisms involving aerobic and anaerobic cultures and the use of different culture media, and including (if performed): (a) pathogen identification and antibiotic susceptibility testing; or (b) a service described in item 69300, 69303, 69306, 69312 or 69318; specimens from one or more sites | 48.15 |
69324 | Microscopy (with appropriate stains) and culture for mycobacteria—one specimen of sputum, urine or other body fluid or one operative or biopsy specimen, including (if performed): (a) microscopy and culture of other bacterial pathogens isolated as a result of this procedure; or (b) pathogen identification and antibiotic susceptibility testing; including a service mentioned in item 69300 | 43.00 |
69325 | A service described in item 69324 if the microscopy and culture is performed by a receiving APP | 43.00 |
69327 | Microscopy (with appropriate stains) and culture for mycobacteria—2 specimens of sputum, urine or other body fluids or operative or biopsy specimens, including (if performed): (a) microscopy and culture of other bacterial pathogens isolated as a result of this procedure; or (b) pathogen identification and antibiotic susceptibility testing; including a service described in item 69300 | 85.00 |
69328 | A service described in item 69327 if the microscopy and culture is performed by a receiving APP | 85.00 |
69330 | Microscopy (with appropriate stains) and culture for mycobacteria—3 specimens of sputum, urine or other body fluids or operative or biopsy specimens, including (if performed): (a) microscopy and culture of other bacterial pathogens isolated as a result of this procedure; or (b) pathogen identification and antibiotic susceptibility testing; including a service described in item 69300 | 128.00 |
69331 | A service described in item 69330 if the microscopy and culture is performed by a receiving APP | 128.00 |
69333 | Urine examination (including serial examination) by any means other than simple culture by dip slide, including: (a) cell count; and (b) culture; and (c) colony count; and (d) (if performed) stained preparations; and (e) (if performed) identification of cultured pathogens; and (f) (if performed) antibiotic susceptibility testing; and (g) (if performed) examination for pH, specific gravity, blood, protein, urobilinogen, sugar, acetone or bile salts | 20.55 |
69336 | Microscopy of faeces for ova, cysts and parasites, that includes the use of: (a) a concentration technique; and (b) fixed stains or antigen detection for cryptosporidia and giardia; and includes a service mentioned in item 69300 (if performed) | 33.45 |
69339 | Microscopy of faeces for ova, cysts and parasites using concentration techniques examined after examination described in item 69336 performed on a separately collected and identified specimen collected within 7 days of the examination described in item 69336—not more than one examination in a 7 day period | 19.10 |
69345 | Culture and (if performed) microscopy without concentration techniques of faeces for faecal pathogens, using at least 2 selective or enrichment media and culture in at least 2 different atmospheres including (if performed): (a) pathogen identification and antibiotic susceptibility testing; and (b) the detection of clostridial toxins; and (c) a service described in item 69300; not more than one examination in a 7 day period | 52.90 |
69354 | Blood culture for pathogenic micro‑organisms (other than viruses), including sub‑cultures and (if performed): (a) identification of any cultured pathogen; and (b) necessary antibiotic susceptibility testing; to a maximum of 3 sets of cultures—one set of cultures | 30.75 |
69357 | Two sets of cultures described in item 69354 | 61.45 |
69360 | Three sets of cultures described in item 69354 | 92.20 |
69363 | Detection of Clostridium difficile or Clostridium difficile toxin (except if a service described in item 69345 has been performed)—one or more tests | 28.65 |
69378 | Quantitation of HIV viral RNA load in plasma or serum in the monitoring of a HIV sero‑positive patient not on antiretroviral therapy—one or more tests | 180.25 |
69379 | A test described in item 69378 if the quantitation is performed by a receiving APP—one or more tests on one or more specimens | 180.25 |
69380 | Genotypic testing for HIV antiretroviral resistance in a patient with confirmed HIV infection if the patient’s viral load is greater than 1 000 copies per ml at any of the following times: (a) at presentation; (b) before antiretroviral therapy; (c) when treatment with combination antiretroviral agents fails; maximum of 2 tests in a 12 month period | 770.30 |
69381 | Quantitation of HIV viral RNA load in plasma or serum in the monitoring of a HIV sero‑positive patient on antiretroviral therapy—one or more tests on one or more specimens | 180.25 |
69382 | Quantitation of HIV viral RNA load in cerebrospinal fluid in a HIV sero‑positive patient—one or more tests on one or more specimens | 180.25 |
69383 | A test described in item 69381 if the quantitation is performed by a receiving APP—one or more tests on one or more specimens | 180.25 |
69384 | Quantitation of one antibody to microbial antigens not elsewhere described in this table—one test | 15.65 |
69387 | Two tests described in item 69384 | 29.00 |
69390 | Three tests described in item 69384 | 42.35 |
69393 | Four tests described in item 69384 | 55.70 |
69396 | Five or more tests described in item 69384 | 69.10 |
69400 | A test described in item 69384 if rendered by a receiving APP, where no tests in the item have been rendered by the referring APP—one test | 15.65 |
69401 | A test described in item 69384 if a referring APP has performed a test or tests described in item 69384—each test to a maximum of 4 tests | 13.35 |
69405 | Microbiological serology during a pregnancy (except in the investigation of a clinically apparent intercurrent microbial illness or close contact with a patient suffering from parvovirus infection or varicella during that pregnancy) including: (a) the determination of one of the following: rubella immune status, specific syphilis serology, carriage of Hepatitis B, Hepatitis C antibody, HIV antibody; and (b) (if performed) a service described in one or more of items 69384, 69475, 69478 and 69481 | 15.65 |
69408 | Microbiological serology during a pregnancy (except in the investigation of a clinically apparent intercurrent microbial illness or close contact with a patient suffering from parvovirus infection or varicella during that pregnancy) including: (a) the determination of 2 of the following: rubella immune status, specific syphilis serology, carriage of Hepatitis B, Hepatitis C antibody, HIV antibody; and (b) (if performed) a service described in one or more of items 69384, 69475, 69478 and 69481 | 29.00 |
69411 | Microbiological serology during a pregnancy (except in the investigation of a clinically apparent intercurrent microbial illness or close contact with a patient suffering from parvovirus infection or varicella during that pregnancy) including: (a) the determination of 3 of the following: rubella immune status, specific syphilis serology, carriage of Hepatitis B, Hepatitis C antibody, HIV antibody; and (b) (if performed) a service described in one or more of items 69384, 69475, 69478 and 69481 | 42.35 |
69413 | Microbiological serology during a pregnancy (except in the investigation of a clinically apparent intercurrent microbial illness or close contact with a patient suffering from parvovirus infection or varicella during that pregnancy) including: (a) the determination of 4 of the following: rubella immune status, specific syphilis serology, carriage of Hepatitis B, Hepatitis C antibody, HIV antibody; and (b) (if performed) a service described in one or more of items 69384, 69475, 69478 and 69481 | 55.70 |
69415 | Microbiological serology during a pregnancy (except in the investigation of a clinically apparent intercurrent microbial illness or close contact with a patient suffering from parvovirus infection or varicella during that pregnancy) including: (a) the determination of all of the following: rubella immune status, specific syphilis serology, carriage of hepatitis B, hepatitis C antibody, HIV antibody; and (b) (if performed) a service described in one or more of items 69384, 69475, 69478 and 69481 | 69.10 |
69418 | A test for high risk human papillomaviruses (HPV) in a patient who: (a) within the 2 year period before the test, has received excisional or ablative treatment for high grade squamous intraepithelial lesions (HSIL) of the cervix; or (b) within the 2 year period before the test, has had a positive HPV test after excisional or ablative treatment for HSIL of the cervix; or (c) is undergoing annual cytological review following treatment for HSIL of the cervix; one test | 63.55 |
69419 | A test described in item 69418 if the test is performed by a receiving APP—one test | 63.55 |
69445 | Detection of hepatitis C viral RNA in a patient undertaking antiviral therapy for chronic HCV hepatitis (including a service described in item 69499)—one test | 92.20 |
69451 | A test described in item 69445 if the test is performed by a receiving APP—one test | 92.20 |
69471 | Test of cell‑mediated immunity in blood for the detection of latent tuberculosis in an immunosuppressed or immunocompromised patient—one test | 34.90 |
69472 | Detection of antibodies to Epstein Barr Virus using specific serology—one test | 15.65 |
69474 | Detection of antibodies to Epstein Barr Virus using specific serology—2 or more tests | 28.65 |
69475 | Detection of hepatitis antigens or antibodies to determine immune status or viral carriage following exposure or vaccination to Hepatitis A, Hepatitis B, Hepatitis C or Hepatitis D—one test | 15.65 |
69478 | Two tests described in item 69475 | 29.25 |
69481 | Investigation of infectious causes of acute or chronic hepatitis—3 tests described in item 69475 | 40.55 |
69482 | Quantitation of hepatitis B viral DNA in patients who are hepatitis B surface antigen positive and have chronic hepatitis B but are not receiving antiviral therapy—one test | 152.10 |
69483 | Quantitation of hepatitis B viral DNA in patients who: (a) are hepatitis B surface antigen positive; and (b) have chronic hepatitis B; and (c) are receiving antiviral therapy; one test | 152.10 |
69484 | Supplementary test for hepatitis B surface antigen or hepatitis C antibody using a different assay on a specimen that yielded a reactive result on initial testing | 17.10 |
69488 | Quantitation of HCV RNA load in plasma or serum in: (a) the pre‑treatment evaluation, of a patient with chronic HCV hepatitis, for antiviral therapy; or (b) the assessment of efficacy of antiviral therapy for such a patient; if the test is requested by, or on the advice of, the specialist or consultant physician who manages the treatment of the patient (including a service described in item 69445 or 69499) | 180.25 |
69489 | A test described in item 69488 if the test is performed by a receiving APP | 180.25 |
69491 | Nucleic acid amplification and determination of hepatitis C virus (HCV) genotype if: (a) the patient is HCV RNA positive and is being evaluated for antiviral therapy of chronic HCV hepatitis; and (b) the request for the test is made by, or on the advice of, the specialist or consultant physician managing the treatment of the patient | 204.80 |
69492 | A service described in item 69491 if the test is performed by a receiving APP | 204.80 |
69494 | Detection of a virus, microbial antigen or microbial nucleic acid (not elsewhere described in this table)—one test | 28.65 |
69495 | Two tests described in item 69494 | 35.85 |
69496 | Three or more tests described in item 69494 | 43.05 |
69497 | This item applies to a test described in item 69494 if: (a) a referring APP has not performed the test described in item 69494; and (b) a receiving APP performs the test described in item 69494; one test | 28.65 |
69498 | This item applies to a test described in item 69494 if: (a) a referring APP has performed the test or tests described in item 69494; and (b) a receiving APP has performed the test or tests described in item 69494; one test | 7.20 |
69499 | Detection of hepatitis C viral RNA if at least one of the following criteria is satisfied: (a) the patient is hepatitis C sero‑positive; (b) the patient’s serological status is uncertain after testing; (c) the test is performed for the purpose of: (i) determining the hepatitis C status of an immunosuppressed or immunocompromised patient; or (ii) the detection of acute hepatitis C prior to seroconversion where considered necessary for the clinical management of the patient | 92.20 |
69500 | A test described in item 69499 if the test is performed by a receiving APP | 92.20 |
Division 2.4—Group P4: immunology
2.4.1 Limitation on certain items
(1) For any particular patient, items 71075, 71127, 71135 and 71137 are applicable not more than twice in a 12 month period.
(2) For any particular patient, item 71079 is applicable not more than 4 times in a 12 month period.
(3) For any particular patient, item 71077 is applicable not more than 6 times in a 12 month period.
2.4.2 Tests in Group P4 relating to antibodies
For items 71119, 71121, 71123 and 71125, if:
(a) tests are carried out in relation to a patient episode; and
(b) specimen material from the patient episode is stored; and
(c) in response to a request made within 14 days of the patient episode, further tests are carried out on the stored material;
the later tests and the earlier tests are taken to be part of one patient episode.
2.4.3 HLA‑B27 typing
If a pathology service mentioned in item 71148 is rendered as a pathologist‑determinable service, the amount mentioned in the item is not payable for a pathology service mentioned in the item unless the recognised pathologist who renders the service records, in writing, the reasons for rendering the service and the result of the pathology service mentioned in item 71147.
2.4.4 Antineutrophil cytoplasmic antibody tests
For subsection 16A(3) of the Act, a request for an antineutrophil cytoplasmic antibody immunofluorescence test is taken to include a request for an antineutrophil proteinase 3 antibody test and an antimyeloperoxidase antibody test if:
(a) the immunofluorescence test performed as a result of the request is abnormal; or
(b) a previous immunofluorescence test was abnormal; or
(c) those antibodies have been previously detected.
Group P4—Immunology |
Item | Pathology service | Fee ($) |
71057 | Electrophoresis, quantitative and qualitative, of serum, urine or other body fluid, collected in a 28 day period, to demonstrate: (a) protein classes; or (b) presence and amount of paraprotein; including the preliminary quantitation of total protein, albumin and globulin—one specimen type | 32.90 |
71058 | Examination as described in item 71057—2 or more specimen types | 50.50 |
71059 | Immunofixation, immunoelectrophoresis or isoelectric focusing of: (a) urine for detection of Bence Jones proteins; or (b) serum, plasma, or other body fluid; and characterisation of a paraprotein or cryoglobulin—examination of one specimen type (e.g. serum, urine or CSF) | 35.65 |
71060 | Examination as described in item 71059 of 2 or more specimen types | 44.05 |
71062 | Electrophoresis and immunofixation or immunoelectrophoresis or isoelectric focusing of CSF for the detection of oligoclonal bands and including if required electrophoresis of the patient’s serum for comparison purposes—one or more tests | 44.05 |
71064 | Detection and quantitation of cryoglobulins or cryofibrinogen—one or more tests | 20.75 |
71066 | Quantitation of total immunoglobulin A (by any method) in serum, urine, or other body fluid—one test | 14.55 |
71068 | Quantitation of total immunoglobulin G (by any method) in serum, urine, or other body fluid—one test | 14.55 |
71069 | Two tests described in item 71066, 71068, 71072 or 71074 | 22.75 |
71071 | Three or more tests described in item 71066, 71068, 71072 or 71074 | 30.95 |
71072 | Quantitation of total immunoglobulin M (by any method) in serum, urine, or other body fluid—one test | 14.55 |
71073 | Quantitation of all 4 immunoglobulin G subclasses | 106.15 |
71074 | Quantitation of total immunoglobulin D (by any method) in serum, urine, or other body fluid—one test | 14.55 |
71075 | Quantitation of immunoglobulin E (total)—one test | 23.00 |
71076 | A test described in item 71073 if the test is performed by a receiving APP—one test | 106.15 |
71077 | Quantitation of immunoglobulin E (total) in the follow up of a patient with proven immunoglobulin‑E‑secreting myeloma, proven congenital immunodeficiency or proven allergic bronchopulmonary aspergillosis—one test | 27.05 |
71079 | Detection of specific immunoglobulin E antibodies to single or multiple potential allergens | 26.80 |
71081 | Quantitation of total haemolytic complement | 40.55 |
71083 | Quantitation of complement components C3 and C4 or properdin factor B—one test | 20.15 |
71085 | Two tests described in item 71083 | 28.95 |
71087 | Three or more tests described in item 71083 | 37.70 |
71089 | Quantitation of complement components or breakdown products of complement proteins not elsewhere described in an item in this table—one test | 29.15 |
71090 | This item applies to a test described in item 71089 if: (a) a referring APP has not performed the test described in item 71089; and (b) a receiving APP performs the test described in item 71089; one test | 29.15 |
71091 | Two tests described in item 71089 | 52.85 |
71092 | This item applies to a test described in item 71089 if: (a) a referring APP has performed the test or tests described in item 71089; and (b) a receiving APP performs the test or tests described in item 71089; one test | 23.70 |
71093 | Three or more tests described in item 71089 | 76.45 |
71095 | Quantitation of serum or plasma eosinophil cationic protein, or both, to a maximum of 3 assays in a 12 month period, for monitoring the response to therapy in corticosteroid treated asthma, in a child aged less than 12 years | 40.55 |
71096 | A test described in item 71095 if the quantitation is performed by a receiving APP | 40.55 |
71097 | Antinuclear antibodies—detection in serum or other body fluids, including quantitation if required | 24.45 |
71099 | Double‑stranded DNA antibodies—quantitation by one or more methods other than the Crithidia method | 26.50 |
71101 | Antibodies to one or more extractable nuclear antigens—detection in serum or other body fluids | 17.40 |
71103 | Characterisation of an antibody detected in a service described in item 71101 (including that service) | 52.05 |
71106 | Rheumatoid factor—detection by any technique in serum or other body fluids, including quantitation if required | 11.30 |
71119 | Antibodies to tissue antigens not elsewhere specified in this table—detection of one antibody, including quantitation if required | 17.35 |
71121 | Detection of 2 antibodies specified in item 71119 | 20.80 |
71123 | Detection of 3 antibodies specified in item 71119 | 24.25 |
71125 | Detection of 4 or more antibodies specified in item 71119 | 27.65 |
71127 | Functional tests for lymphocytes—quantitation, other than by microscopy, of: (a) proliferation induced by one or more mitogens; or (b) proliferation induced by one or more antigens; or (c) estimation of one or more mixed lymphocyte reactions; including a test described in item 65066 or 65070 (if performed) | 176.35 |
71129 | Two tests described in item 71127 | 217.85 |
71131 | Three or more tests described in item 71127 | 259.35 |
71133 | Investigation of recurrent infection, by qualitative assessment, for the presence of defects in oxidative pathways in neutrophils by the nitroblue tetrazolium (NBT) reduction test | 10.40 |
71134 | Investigation of recurrent infection, by quantitative assessment, of oxidative pathways by flow cytometric techniques, including a test described in item 71133 (if performed) | 104.05 |
71135 | Quantitation of neutrophil function, comprising at least 2 of the following: (a) chemotaxis; (b) phagocytosis; (c) oxidative metabolism; (d) bactericidal activity; including any test described in item 65066, 65070, 71133 or 71134 (if performed) | 207.95 |
71137 | Quantitation of cell‑mediated immunity by multiple antigen delayed type hypersensitivity intradermal skin testing using a minimum of 7 antigens | 30.25 |
71139 | Characterisation of 3 or more leucocyte surface antigens by immunofluorescence or immunoenzyme techniques to assess lymphoid or myeloid cell populations, including a total lymphocyte count or total leucocyte count by any method, on one or more specimens of blood, CSF or serous fluid | 104.05 |
71141 | Characterisation of 3 or more leucocyte surface antigens by immunofluorescence or immunoenzyme techniques to assess lymphoid or myeloid cell populations on one or more disaggregated tissue specimens | 197.35 |
71143 | Characterisation of 6 or more leucocyte surface antigens by immunofluorescence or immunoenzyme techniques to assess lymphoid or myeloid cell populations for the diagnosis (but not monitoring) of an immunological or haematological malignancy, including a service described in one or both of items 71139 and 71141 (if performed), on a specimen of blood, CSF, serous fluid or disaggregated tissue | 260.00 |
71145 | Characterisation of 6 or more leucocyte surface antigens by immunofluorescence or immunoenzyme techniques to assess lymphoid or myeloid cell populations for the diagnosis (but not monitoring) of an immunological or haematological malignancy, including a service described in one or more of items 71139, 71141 and 71143 (if performed) on 2 or more specimens of disaggregated tissues or one specimen of disaggregated tissue and one or more specimens of blood, CSF or serous fluid | 424.50 |
71146 | Enumeration of CD34+ cells, only for the purposes of autologous or directed allogeneic haemopoietic stem cell transplantation, including a total white cell count on the pheresis collection | 104.05 |
71147 | HLA‑B27 typing | 40.55 |
71148 | A test described in item 71147 if a receiving APP performs the test | 40.55 |
71149 | Complete tissue typing for 4 HLA‑A and HLA‑B Class I antigens (including any separation of leucocytes), including (if performed) a service described in item 71147 | 108.25 |
71151 | Tissue typing for HLA‑DR, HLA‑DP and HLA‑DQ Class II antigens (including any separation of leucocytes)—phenotyping or genotyping of 2 or more antigens | 118.85 |
71153 | Testing, for assessment or diagnosis of systemic inflammatory disease or vasculitis, for the presence of an antibody by one of the following tests: (a) antineutrophil cytoplasmic antibody (ANCA) immunofluorescence test; (b) antineutrophil proteinase 3 antibody (PR3 ANCA) test; (c) antimyeloperoxidase antibody (MPO ANCA) test; (d) antiglomerular basement membrane antibody (GBM ANCA) test | 34.55 |
71154 | This item applies to a test described in item 71153 if: (a) a referring APP has performed a test or tests described in item 71153; and (b) a receiving APP performs the test described in item 71153; one test | 34.55 |
71155 | Testing for the presence of 2 antibodies by tests mentioned in item 71153 | 47.45 |
71156 | This item applies to a test described in item 71153 (other than a test described in item 71154) if: (a) a referring APP has performed the test or tests described in item 71153; and (b) a receiving APP performs the test or tests described in item 71153; one test | 12.85 |
71157 | Testing for the presence of 3 antibodies by tests mentioned in item 71153 | 60.30 |
71159 | Testing for the presence of 4 antibodies by tests mentioned in item 71153 | 73.15 |
71163 | Detection of one of the following antibodies (of one or more class or isotype) in the assessment or diagnosis of coeliac disease or other gluten hypersensitivity syndromes, including a service described in item 71066 (if performed): (a) antibodies to gliadin; (b) antibodies to endomysium; (c) antibodies to tissue transglutaminase; one test | 24.75 |
71164 | Two or more tests mentioned in item 71163, including a service described in item 71066 (if performed) | 39.90 |
71165 | Antibodies to tissue antigens (acetylcholine receptor, adrenal cortex, heart, histone, insulin, insulin receptor, intrinsic factor, islet cell, lymphocyte, neuron, ovary, parathyroid, platelet, salivary gland, skeletal muscle, skin basement membrane and intercellular substance, thyroglobulin, thyroid microsome or thyroid stimulating hormone receptor)—detection of one antibody, including quantitation if required | 34.55 |
71166 | Detection of 2 antibodies described in item 71165 | 47.45 |
71167 | Detection of 3 antibodies described in item 71165 | 60.30 |
71168 | Detection of 4 or more antibodies described in item 71165 | 73.15 |
71169 | This item applies to a service described in item 71165 if: (a) a referring APP has not performed the service described in item 71165; and (b) a receiving APP performs the service described in item 71165 | 34.55 |
71170 | This item applies to a service described in item 71165 if: (a) a referring APP has performed the test or tests described in item 71165; and (b) a receiving APP performs the test or tests described in item 71165; one test | 12.85 |
71180 | Antibody to cardiolipin or beta‑2 glycoprotein I—detection, including quantitation if required; one antibody specificity (IgG or IgM) | 34.55 |
71183 | Detection of 2 antibodies described in item 71180 | 47.45 |
71186 | Detection of 3 or more antibodies described in item 71180 | 60.30 |
71189 | Detection of specific IgG antibodies to one or more respiratory disease allergens not elsewhere specified | 15.50 |
71192 | Two items described in item 71189 | 28.35 |
71195 | Three or more items described in item 71189 | 40.05 |
71198 | Estimation of serum tryptase for the evaluation of unexplained acute hypotension or suspected anaphylactic event, assessment of risk in stinging insect anaphylaxis, exclusion of mastocytosis, monitoring of known mastocytosis | 40.55 |
71200 | Detection and quantitation, if present, of free kappa and lambda light chains in serum for the diagnosis or monitoring of amyloidosis, myeloma or plasma cell dyscrasias | 59.60 |
71203 | Determination of HLAB5701 status by flow cytometry or cytotoxity assay prior to the initiation of Abacavir therapy including item 73323 (if performed) | 40.55 |
Division 2.5—Group P5: tissue pathology
2.5.1 Tests on biopsy material
(1) For items in Group P5 (Tissue pathology):
biopsy material means all tissue received by an approved pathology practitioner:
(a) from a medical procedure, or group of medical procedures, performed on a patient at the same time; or
(b) after being expelled spontaneously from a patient.
cytology means microscopic examination of one or more stained preparations of cells separated naturally or artificially from their normal environment by methods recognised as adequate to demonstrate their structure to a degree sufficient to enable an opinion to be formed about whether they are likely to be normal, abnormal but benign, or abnormal and malignant but, in accordance with customary laboratory practice, does not include examination of a blood film or a bone marrow aspirate.
separately identified specimen means an individual specimen collected, identified so that it is clearly distinguished from any other specimen, and sent for testing by or on behalf of the treating practitioner responsible for the procedure in which the specimen was taken.
(2) For Group P5, services in Group P6 (Cytology) include any services described in Group P5 on the material submitted for a test in Group P6.
(3) For subclause (2), any sample submitted for cytology from which a cell block is prepared does not qualify for a Group P5 item.
(4) If more than one of the services mentioned in items 72813, 72816, 72817, 72818, 72823, 72824, 72825, 72826, 72827, 72828, 72830, 72836 and 72838 are performed in a single patient episode, only the fee for the item performed having the highest specified fee is applicable to the services.
(5) If more than one histopathological examination is performed on separate specimens, of different complexity levels, from a single patient episode, only the fee for the examination having the highest specified fee is applicable to the examinations.
(6) In items 72813, 72816, 72817, 72818, 72823, 72824, 72825, 72826, 72827, 72828, 72830, 72836 and 72838 a reference to a complexity level is a reference to the level given to a specimen type mentioned in Part 4 of this table.
(7) If:
(a) more than one of the services mentioned in items 72846, 72847, 72848, 72849 and 72850; or
(b) more than one of the services mentioned in items 73059, 73060, 73061, 73064 and 73065;
are performed in a single patient episode, only the fee for the item performed having the higher or highest specified fee applies to the services.
Group P5—Tissue pathology |
Item | Pathology service | Fee ($) |
72813 | Examination of complexity level 2 biopsy material with one or more tissue blocks, including specimen dissection, all tissue processing, staining, light microscopy and professional opinion or opinions—one or more separately identified specimens | 71.50 |
72816 | Examination of complexity level 3 biopsy material with one or more tissue blocks, including specimen dissection, all tissue processing, staining, light microscopy and professional opinion or opinions—one separately identified specimen | 86.35 |
72817 | Examination of complexity level 3 biopsy material with one or more tissue blocks, including specimen dissection, all tissue processing, staining, light microscopy and professional opinion or opinions—2 to 4 separately identified specimens | 96.80 |
72818 | Examination of complexity level 3 biopsy material with one or more tissue blocks, including specimen dissection, all tissue processing, staining, light microscopy and professional opinion or opinions—5 or more separately identified specimens | 107.05 |
72823 | Examination of complexity level 4 biopsy material with one or more tissue blocks, including specimen dissection, all tissue processing, staining, light microscopy and professional opinion or opinions—one separately identified specimen | 97.15 |
72824 | Examination of complexity level 4 biopsy material with one or more tissue blocks, including specimen dissection, all tissue processing, staining, light microscopy and professional opinion or opinions—2 to 4 separately identified specimens | 141.35 |
72825 | Examination of complexity level 4 biopsy material with one or more tissue blocks, including specimen dissection, all tissue processing, staining, light microscopy and professional opinion or opinions—5 to 7 separately identified specimens | 180.25 |
72826 | Examination of complexity level 4 biopsy material with one or more tissue blocks, including specimen dissection, all tissue processing, staining, light microscopy and professional opinion or opinions—8 to 11 separately identified specimens | 194.60 |
72827 | Examination of complexity level 4 biopsy material with one or more tissue blocks, including specimen dissection, all tissue processing, staining, light microscopy and professional opinion or opinions—12 to 17 separately identified specimens | 208.95 |
72828 | Examination of complexity level 4 biopsy material with one or more tissue blocks, including specimen dissection, all tissue processing, staining, light microscopy and professional opinion or opinions—18 or more separately identified specimens | 223.30 |
72830 | Examination of complexity level 5 biopsy material with one or more tissue blocks, including specimen dissection, all tissue processing, staining, light microscopy and professional opinion or opinions—one or more separately identified specimens | 274.15 |
72836 | Examination of complexity level 6 biopsy material with one or more tissue blocks, including specimen dissection, all tissue processing, staining, light microscopy and professional opinion or opinions—one or more separately identified specimens | 417.20 |
72838 | Examination of complexity level 7 biopsy material with multiple tissue blocks, including specimen dissection, all tissue processing, staining, light microscopy, and professional opinion or opinions—one or more separately identified specimens | 466.85 |
72844 | Enzyme histochemistry of skeletal muscle for investigation of primary degenerative or metabolic muscle diseases or of muscle abnormalities secondary to disease of the central or peripheral nervous system—one or more tests | 30.75 |
72846 | Immunohistochemical examination of biopsy material by immunofluorescence, immunoperoxidase or other labelled antibody techniques with multiple antigenic specificities per specimen—one to 3 antibodies except those mentioned in item 72848 | 59.60 |
72847 | Immunohistochemical examination of biopsy material by immunofluorescence, immunoperoxidase or other labelled antibody techniques with multiple antigenic specificities per specimen—4 to 6 antibodies | 89.40 |
72848 | Immunohistochemical examination of biopsy material by immunofluorescence, immunoperoxidase or other labelled antibody techniques with multiple antigenic specificities per specimen—one to 3 of the following antibodies: (a) oestrogen; (b) progesterone; (c) c‑erb‑B2 (HER2) | 74.50 |
72849 | Immunohistochemical examination of biopsy material by immunofluorescence, immunoperoxidase or other labelled antibody techniques with multiple antigenic specificities per specimen—7 to 10 antibodies | 104.30 |
72850 | Immunohistochemical examination of biopsy material by immunofluorescence, immunoperoxidase or other labelled antibody techniques with multiple antigenic specificities per specimen—11 or more antibodies | 119.20 |
72851 | Electron microscopic examination of biopsy material—one separately identified specimen | 184.35 |
72852 | Electron microscopic examination of biopsy material—2 or more separately identified specimens | 245.80 |
72855 | Intraoperative consultation and examination of biopsy material by frozen section or tissue imprint or smear—one separately identified specimen | 184.35 |
72856 | Intraoperative consultation and examination of biopsy material by frozen section or tissue imprint or smear—2 to 4 separately identified specimens | 245.80 |
72857 | Intraoperative consultation and examination of biopsy material by frozen section or tissue imprint or smear—5 or more separately identified specimens | 286.75 |
Division 2.6—Group P6: cytology
2.6.1 Tests on biopsy material
(1) For Group P6 (Cytology), services in Group P6 include any services described in Group P5 (Tissue pathology) on the material submitted for a test in Group P6.
(2) For subclause (1), any sample submitted for cytology from which a cell block is prepared does not qualify for a Group P5 item.
(3) If:
(a) more than one of the services mentioned in items 72846, 72847, 72848, 72849 and 72850; or
(b) more than one of the services mentioned in items 73059, 73060, 73061, 73064 and 73065;
are performed in a single patient episode, only the fee for the item performed having the higher or highest specified fee applies to the services.
(4) If more than one of the services mentioned in items 73049, 73051, 73062, 73063, 73066 and 73067 are performed in a single patient episode, the fee for the combined services is:
(a) if services mentioned in 2 items are performed—the higher of the 2 fees specified; or
(b) if services mentioned in more than 2 items are performed—the highest of the fees specified.
Group P6—Cytology |
Item | Pathology service | Fee ($) |
73043 | Cytology (including serial examinations) of nipple discharge or smears from skin, lip, mouth, nose or anus for detection of precancerous or cancerous changes—one or more tests | 22.85 |
73045 | Cytology (including serial examinations) for malignancy (other than an examination mentioned in item 73053), including any Group P5 service (if performed), one or more tests on: (a) specimens resulting from washings or brushings from sites not specified in item 73043; or (b) a single specimen of sputum or urine; or (c) one or more specimens of other body fluids | 48.60 |
73047 | Cytology of a series of 3 sputum or urine specimens for malignant cells | 94.70 |
73049 | Cytology of material obtained directly from a patient by fine needle aspiration of solid tissue, or tissues—one identified site | 68.15 |
73051 | Cytology of material obtained directly from a patient at one identified site by fine needle aspiration of solid tissue or tissues if a recognised pathologist: (a) performs the aspiration; or (b) attends the aspiration and performs a cytological examination during the attendance | 170.35 |
73053 | Cytology of a smear from cervix, where the smear is prepared by direct application of the specimen to a slide, excluding the use of liquid based slide preparation techniques, and the stained smear is microscopically examined by or on behalf of a pathologist—each examination: (a) for the detection of precancerous or cancerous changes in women with no symptoms, signs or recent history suggestive of cervical neoplasia; or (b) if a further specimen is taken due to an unsatisfactory smear taken for the purposes of paragraph (a); or (c) if there is inadequate information provided to use item 73055 | 19.45 |
73055 | Cytology of a smear from cervix, not associated with item 73053, where the smear is prepared by direct application of the specimen to a slide, excluding the use of liquid based slide preparation techniques, and the stained smear is microscopically examined by or on behalf of a pathologist—each test: (a) for the management of previously detected abnormalities including precancerous or cancerous conditions; or (b) for the investigation of women with symptoms, signs or recent history suggestive of cervical neoplasia | 19.45 |
73057 | Cytology of a smear from vagina, not associated with item 73053 or 73055, and not to monitor hormone replacement therapy, where the smear is prepared by direct application of the specimen to a slide, excluding the use of liquid based slide preparation techniques, and the stained smear is microscopically examined by or on behalf of a pathologist—each test | 19.45 |
73059 | Immunocytochemical examination of material obtained by procedures described in items 73045, 73047, 73049, 73051, 73062 73063, 73066 and 73067 for the characterisation of a malignancy by immunofluorescence, immunoperoxidase or other labelled antibody techniques with multiple antigenic specificities per specimen—one to 3 antibodies except those mentioned in item 73061 | 43.00 |
73060 | Immunocytochemical examination of material obtained by procedures described in items 73045, 73047, 73049, 73051, 73062 73063, 73066 and 73067 for the characterisation of a malignancy by immunofluorescence, immunoperoxidase or other labelled antibody techniques with multiple antigenic specificities per specimen—4 to 6 antibodies | 57.35 |
73061 | Immunocytochemical examination of material obtained by procedures described in items 73045, 73047, 73049, 73051, 73062 73063, 73066 and 73067 for the characterisation of a malignancy by immunofluorescence, immunoperoxidase or other labelled antibody techniques with multiple antigenic specificities per specimen—one to 3 of the following antibodies: (a) oestrogen; (b) progesterone; (c) c‑erb‑B2 (HER2) | 51.20 |
73062 | Cytology of material obtained directly from a patient by fine needle aspiration of solid tissue, or tissues—2 or more separately identified sites | 89.00 |
73063 | Cytology of material obtained directly from a patient at one identified site by fine needle aspiration of solid tissue, or tissues, if an employee of an approved pathology authority attends the aspiration for confirmation of sample adequacy | 99.35 |
73064 | Immunocytochemical examination of material obtained by procedures described in items 73045, 73047, 73049, 73051, 73062 73063, 73066 and 73067 for the characterisation of a malignancy by immunofluorescence, immunoperoxidase or other labelled antibody techniques with multiple antigenic specificities per specimen—7 to 10 antibodies | 71.70 |
73065 | Immunocytochemical examination of material obtained by procedures described in items 73045, 73047, 73049, 73051, 73062 73063, 73066 and 73067 for the characterisation of a malignancy by immunofluorescence, immunoperoxidase or other labelled antibody techniques with multiple antigenic specificities per specimen—11 or more antibodies | 86.00 |
73066 | Cytology of material obtained directly from a patient at 2 or more separately identified sites by fine needle aspiration of solid tissue, or tissues, if a recognised pathologist: (a) performs the aspiration; or (b) attends the aspiration and performs cytological examination during the attendance | 221.45 |
73067 | Cytology of material obtained directly from a patient at 2 or more separately identified sites by fine needle aspiration of solid tissue, or tissues, if an employee of an approved pathology authority attends the aspiration for confirmation of sample adequacy | 129.15 |
Division 2.7—Group P7: genetics
2.7.1 Haemochromatosis testing
For items 73317 and 73318:
elevated serum ferritin, for a patient, means a level of ferritin above the normal reference range for the particular method of assay used to determine the level.
2.7.2 HLA‑B27 typing
If a pathology service mentioned in item 73320 or 73321 is rendered as a pathologist‑determinable service, the amount mentioned in the item is not payable for a pathology service mentioned in the item unless the recognised pathologist who renders the service records, in writing, the reasons for rendering the service and the result of the pathology service mentioned in item 71147.
2.7.3 Limitation on items 73339 and 73340
For any particular patient, each of items 73339 and 73340 is applicable only once.
Group P7—Genetics |
Item | Pathology service | Fee ($) |
73287 | Study of the whole of every chromosome by cytogenetics or other techniques, performed on one or more of any tissue or fluid except blood (including a service mentioned in item 73293, if performed)—one or more tests | 394.55 |
73289 | Study of the whole of every chromosome by cytogenetics or other techniques, performed on blood (including a service mentioned in item 73293, if performed)—one or more tests | 358.95 |
73290 | Study of the whole of every chromosome by cytogenetics or other techniques, performed on blood or bone marrow, to diagnose or monitor haematological malignancy (including a service mentioned in item 73287 or 73289, if performed)—one or more tests | 394.55 |
73291 | Analysis of one or more chromosome regions, performed on blood or fresh tissue, for specific constitutional genetic abnormalities in: (a) diagnostic studies of a person with developmental delay, intellectual disability, autism, or at least 2 congenital abnormalities, in whom a study by cytogenetics or other techniques mentioned in item 73287 or 73289 is normal or has not been performed—one or more tests; or (b) studies of a relative of the person for an abnormality previously identified in the person—one or more tests | 230.95 |
73292 | Analysis of chromosomes by genome‑wide microarray, including targeted assessment of specific regions for constitutional genetic abnormalities in diagnostic studies of a person with developmental delay, intellectual disability, autism, or at least 2 congenital abnormalities (including a service mentioned in item 73287, 73289 or 73291, if performed)—one or more tests | 589.90 |
73293 | Analysis of one or more regions on all chromosomes, performed on fresh tissue, for specific constitutional genetic abnormalities in diagnostic studies of the products of conception, including exclusion of maternal cell contamination—one or more tests | 230.95 |
73294 | Analysis of the PMP22 gene for constitutional genetic abnormalities causing peripheral neuropathy, as: (a) diagnostic studies of a person with peripheral neuropathy—one or more tests; or (b) studies of a relative of the person for an abnormality previously identified in the person—one or more tests | 230.95 |
73300 | Detection of mutation of the FMR1 gene if: (a) the patient exhibits intellectual disability, ataxia, neurodegeneration, or premature ovarian failure consistent with an FMR1 mutation; or (b) the patient has a relative with an FMR1 mutation; one or more tests | 101.30 |
73305 | Detection of a mutation of the FMR1 gene by Southern Blot analysis, if the results of a service performed in item 73300 are inconclusive | 202.65 |
73308 | Characterisation of the genotype of a patient for Factor V Leiden gene mutation, or detection of other relevant mutations in the investigation of proven venous thrombosis or pulmonary embolism—one or more tests | 36.45 |
73309 | A test described in item 73308 if the test is performed by a receiving APP—one or more tests | 36.45 |
73311 | Characterisation of the genotype of a person who is a first degree relative of a person who has been proven to have one or more abnormal genotypes under item 73308—one or more tests | 36.45 |
73312 | A test described in item 73311 if the test is performed by a receiving APP—one or more tests | 36.45 |
73314 | Characterisation of gene rearrangement or the identification of mutations within a known gene rearrangement in the diagnosis and monitoring of patients with laboratory evidence of: (a) acute myeloid leukaemia; or (b) acute promyelocytic leukaemia; or (c) acute lymphoid leukaemia; or (d) chronic myeloid leukaemia | 230.95 |
73315 | A service described in item 73314 if the characterisation is performed by a receiving APP—one or more tests | 230.95 |
73317 | Detection of the C282Y genetic mutation of the HFE gene and, if performed, detection of other mutations for haemochromatosis where: (a) the patient has an elevated transferrin saturation or elevated serum ferritin on testing of repeated specimens; or (b) the patient has a first degree relative with haemochromatosis; or (c) the patient has a first degree relative with homozygosity for the C282Y genetic mutation, or with compound heterozygosity for recognised genetic mutations for haemochromatosis | 36.45 |
73318 | A test described in item 73317 if the detection is performed by a receiving APP—one or more tests | 36.45 |
73320 | Detection of HLA‑B27 by nucleic acid amplification including a service described in item 71147 unless the service in this item is rendered as a pathologist‑determinable service | 40.55 |
73321 | A test described in item 73320 if the detection is performed by a receiving APP—one or more tests | 40.55 |
73323 | Determination of HLAB5701 status by molecular techniques prior to the initiation of Abacavir therapy including item 71203 (if performed) | 40.55 |
73324 | A test described in item 73323 if rendered by a receiving APP—one or more tests | 40.95 |
73325 | Characterisation of mutations in: (a) the JAK2 gene; or (b) the MPL gene; or (c) both genes; in the diagnostic work‑up, by or on behalf of a specialist or consultant physician, of a patient with clinical and laboratory evidence of: (d) polycythaemia vera; or (e) essential thrombocythaemia; one or more tests | 74.50 |
73326 | Characterisation of the gene rearrangement FIP1L1‑PDGFRA in the diagnostic work‑up and management of a patient with laboratory evidence of: (a) mast cell disease; or (b) idiopathic hypereosinophilic syndrome; or (c) chronic eosinophilic leukaemia; one or more tests | 230.95 |
73327 | Detection of genetic polymorphisms in the Thiopurine S‑methyltransferase gene for the prevention of dose‑related toxicity during treatment with thiopurine drugs, including (if performed) any service described in item 65075—one or more tests | 51.95 |
73332 | An in situ hybridisation (ISH) test of tumour tissue from a patient with breast cancer requested by, or on the advice of, a specialist or consultant physician who manages the treatment of the patient to determine if the requirements relating to human epidermal growth factor receptor 2 (HER2) gene amplification for access to trastuzumab under the Pharmaceutical Benefits Scheme or the Herceptin Program are fulfilled | 315.40 |
73333 | Detection of germline mutations of the von Hippel‑Lindau (VHL) gene: (a) in a patient who has a clinical diagnosis of VHL syndrome and: (i) a family history of VHL syndrome and one of the following: (A) haemangioblastoma (retinal or central nervous system); (B) phaeochromocytoma; (C) renal cell carcinoma; or (ii) 2 or more haemangioblastomas; or (iii) one haemangioblastoma and a tumour or a cyst of: (A) the adrenal gland; or (B) the kidney; or (C) the pancreas; or (D) the epididymis; or (E) a broad ligament (other than epididymal and single renal cysts, which are common in the general population); or (b) in a patient presenting with one or more of the following clinical features suggestive of VHL syndrome: (i) haemangiblastomas of the brain, spinal cord, or retina; (ii) phaeochromocytoma; (iii) functional extra‑adrenal paraganglioma | 600.00 |
73334 | Detection of germline mutations of the von Hippel‑Lindau (VHL) gene in biological relatives of a patient with a known mutation in the VHL gene | 340.00 |
73335 | Detection of somatic mutations of the von Hippel‑Lindau (VHL) gene in a patient with: (a) 2 or more tumours comprising: (i) 2 or more haemangioblastomas, or (ii) one haemangioblastomas and a tumour of: (A) the adrenal gland; or (B) the kidney; or (C) the pancreas; or (D) the epididymis; and (b) no germline mutations of the VHL gene identified by genetic testing | 470.00 |
73336 | A test of tumour tissue from a patient with unresectable stage III or stage IV metastatic cutaneous melanoma, requested by, or on behalf of, a specialist or consultant physician, to determine if the requirements relating to BRAF V600 mutation status for access to dabrafenib under the Pharmaceutical Benefits Scheme are fulfilled | 230.95 |
73337 | A test of tumour tissue from a patient diagnosed with non‑small cell lung cancer, shown to have non‑squamous histology or histology not otherwise specified, requested by, or on behalf of, a specialist or consultant physician, to determine if the requirements relating to epidermal growth factor receptor (EGFR) gene status for access to erlotinib or gefitinib under the Pharmaceutical Benefits Scheme are fulfilled | 397.35 |
73339 | Detection of germline mutations in the RET gene in patients with a suspected clinical diagnosis of multiple endocrine neoplasia type 2 (MEN2) requested by a specialist or consultant physician who manages the treatment of the patient—one test | 400.00 |
73340 | Detection of a known mutation in the RET gene in an asymptomatic relative of a patient with a documented pathogenic germline RET mutation requested by a specialist or consultant physician who manages the treatment of the patient—one test | 200.00 |
Division 2.8—Group P8: infertility and pregnancy tests
2.8.1 Limitation on item 73523
For any particular patient, item 73523 is applicable not more than 4 times in a 12 month period.
Group P8—Infertility and pregnancy tests |
Item | Pathology service | Fee ($) |
73521 | Semen examination for presence of spermatozoa or examination of cervical mucus for spermatozoa (Huhner’s test) | 9.70 |
73523 | Semen examination (other than post‑vasectomy semen examination), including: (a) measurement of volume, sperm count and motility; and (b) examination of stained preparations; and (c) morphology; and (d) (if performed) differential count and one or more chemical tests | 41.75 |
73525 | Sperm antibodies—sperm‑penetrating ability—one or more tests | 28.35 |
73527 | Human chorionic gonadotrophin (HCG)—detection in serum or urine by one or more methods for diagnosis of pregnancy—one or more tests | 10.00 |
73529 | Human chorionic gonadotrophin (HCG), quantitation in serum by one or more methods (except by latex, membrane, strip or other pregnancy test kit) for diagnosis of threatened abortion, or follow up of abortion or diagnosis of ectopic pregnancy, including any services performed in item 73527—one test | 28.65 |
Division 2.9—Group P9: simple basic pathology tests
Group P9—Simple basic pathology tests |
Item | Pathology service | Fee ($) |
73801 | Semen examination for presence of spermatozoa | 6.90 |
73802 | Leucocyte count, erythrocyte sedimentation rate, examination of blood film (including differential leucocyte count), haemoglobin, haematocrit or erythrocyte count—one test | 4.55 |
73803 | Two tests described in item 73802 | 6.35 |
73804 | Three or more tests described in item 73802 | 8.15 |
73805 | Microscopy of urine, whether stained or not, or catalase test | 4.55 |
73806 | Pregnancy test by one or more immunochemical methods | 10.15 |
73807 | Microscopy for wet film other than urine, including any relevant stain | 6.90 |
73808 | Microscopy of Gram‑stained film, including (if performed) a service described in item 73805 or 73807 | 8.65 |
73809 | Chemical tests for occult blood in faeces by reagent stick, strip, tablet or similar method | 2.35 |
73810 | Microscopy for fungi in skin, hair or nails—one or more sites | 6.90 |
73811 | Mantoux test | 11.20 |
Division 2.10—Group P10: patient episode initiation
2.10.1 Items in Group P10 not to apply in certain circumstances
(1) In this Division:
approved collection centre has the meaning given by subsection 23DA(1) of the Act.
institution means a place at which residential accommodation or day care is, or both residential accommodation and day care are, made available to:
(a) disadvantaged children; or
(b) juvenile offenders; or
(c) aged persons; or
(d) chronically ill psychiatric patients; or
(e) homeless persons; or
(f) unemployed persons; or
(g) persons suffering from alcoholism; or
(h) persons addicted to drugs; or
(i) physically or mentally handicapped persons;
but does not include:
(j) a hospital; or
(k) a residential care facility; or
(l) accommodation for aged persons that is attached to a residential care facility or situated within a residential care facility complex.
prescribed laboratory means a laboratory operated by:
(a) the Commonwealth; or
(b) an authority of the Commonwealth; or
(c) a State or internal Territory; or
(d) an authority of a State or internal Territory; or
(e) an Australian tertiary education institution.
residential care facility means a facility in which residential care, within the meaning of section 41‑3 of the Aged Care Act 1997, is provided.
specimen collection centre has the meaning given by subsection 23DA(1) of the Act.
treating practitioner has the same meaning as in paragraph 16A(1)(a) of the Act.
(2) If a service mentioned in an item in Group P10 is rendered by, or on behalf of, an approved pathology practitioner who is a recognised pathologist, the relevant item does not apply to the service if:
(a) the service is rendered upon a request made in the course of an out‑patient service at a recognised hospital; or
(b) the service is rendered to a public patient at a recognised hospital.
(3) An item in Group P10 does not apply to a pathology service to which subsection 16A(7) of the Act applies.
(4) An item in Group P10 does not apply to a pathology service unless at least one item in Groups P1 to P8 also applies to the service.
(5) Subject to subclause (6), if one item in Group P10 applies to a patient episode, no other item in the Group applies to the patient episode.
(6) If, for the same patient episode:
(a) services mentioned in one or more items in Group P5 and one or more of Groups P1, P2, P3, P4, P6, P7 and P8 are rendered by an approved pathology practitioner in the laboratory of another approved pathology authority; or
(b) services mentioned in one or more items in Group P6 and one or more of Groups P1, P2, P3, P4, P5, P7 and P8 are rendered by an approved pathology practitioner in the laboratory of another approved pathology authority;
the fee mentioned in the applicable item in Group P10 applies to both approved pathology practitioners.
(7) If more than one specimen is collected from a person on the same day for the provision of pathology services:
(a) in accordance with more than one request; and
(b) in or by a single approved pathology authority;
the fee mentioned in the applicable item in Group P10 applies once only to the services unless an exemption mentioned in clause 1.2.3, 2.1.1 or 2.2.2 applies or the Minister has made a direction under subsection 4B(3) of the Act.
Group P10—Patient episode initiation |
Item | Pathology service | Fee ($) |
73920 | Initiation of a patient episode by collection of a specimen for one or more services (other than those described in item 73922, 73924 or 73926) if the specimen is collected in an approved collection centre that the approved pathology authority operates in the same premises as it operates a category GX or GY pathology laboratory | 2.40 |
73922 | Initiation of a patient episode that consists of a service described in item 73053, 73055 or 73057 (in circumstances other than those described in item 73923) | 8.20 |
73923 | Initiation of a patient episode that consists of a service described in item 73053, 73055 or 73057 if: (a) the person is a private patient in a recognised hospital; or (b) the person receives the service from a prescribed laboratory | 2.40 |
73924 | Initiation of a patient episode that consists of one or more services described in items 72813, 72816, 72817, 72818, 72823, 72824, 72825, 72826, 72827, 72828, 72830, 72836 and 72838 (in circumstances other than those described in item 73925) from a person who is an inpatient of a hospital | 14.65 |
73925 | Initiation of a patient episode that consists of one or more services described in items 72813, 72816, 72817, 72818, 72823, 72824, 72825, 72826, 72827, 72828, 72830, 72836 and 72838 if the person is: (a) a private patient of a recognised hospital; or (b) a private patient of a hospital who receives the service or services from a prescribed laboratory | 2.40 |
73926 | Initiation of a patient episode that consists of one or more services described in items 72813, 72816, 72817, 72818, 72823, 72824, 72825, 72826, 72827, 72828, 72830, 72836 and 72838 (in circumstances other than those described in item 73927) from a person who is not a patient of a hospital | 8.20 |
73927 | Initiation of a patient episode by a prescribed laboratory that consists of one or more services described in items 72813, 72816, 72817, 72818, 72823, 72824, 72825, 72826, 72827, 72828, 72830, 72836 and 72838 from a person who is not a patient of a hospital | 2.40 |
73928 | Initiation of a patient episode by collection of a specimen for one or more services (in circumstances other than those described in item 73920, 73922, 73923, 73924, 73925, 73926, 73927 or 73929) if the specimen is collected in an approved collection centre | 5.95 |
73929 | Initiation of a patient episode by collection of a specimen for one or more services (in circumstances other than those described in item 73922, 73923, 73924, 73925, 73926 or 73927) if the specimen is collected in an approved collection centre by: (a) an approved pathology practitioner of a prescribed laboratory; or (b) an employee of an approved pathology authority of a prescribed laboratory | 2.40 |
73930 | Initiation of a patient episode by collection of a specimen for one or more services (in circumstances other than those described in item 73922, 73923, 73924, 73925, 73926, 73927 or 73931) if the specimen is collected from a person who is an inpatient of a hospital other than a recognised hospital by an approved pathology practitioner or an employee of an approved pathology authority | 5.95 |
73931 | Initiation of a patient episode by collection of a specimen for one or more services (in circumstances other than those described in item 73922, 73923, 73924, 73925, 73926 or 73927) if the specimen is collected: (a) from a person who is a private patient of a hospital by an approved pathology practitioner of a prescribed laboratory; or (b) from a person who is a private patient of a hospital by an employee of an approved pathology authority that operates a prescribed laboratory; or (c) from a person who is a private patient of a recognised hospital by an approved pathology practitioner of an approved pathology authority; or (d) from a person who is a private patient of a recognised hospital by an employee of an approved pathology authority | 2.40 |
73932 | Initiation of a patient episode by collection of a specimen for one or more services (in circumstances other than those described in item 73922, 73923, 73924, 73925, 73926, 73927 or 73933) if the specimen is collected from a person in the place where the person resides, and that place is not an institution, by: (a) an approved pathology practitioner of an approved pathology authority; or (b) an employee of an approved pathology authority | 10.25 |
73933 | Initiation of a patient episode by collection of a specimen for one or more services (in circumstances other than those described in item 73922, 73923, 73924, 73925, 73926 or 73927) if the specimen is collected from a person in the place where the person resides, and that place is not an institution, by: (a) an approved pathology practitioner of a prescribed laboratory; or (b) an employee of an approved pathology authority that operates a prescribed laboratory | 2.40 |
73934 | Initiation of a patient episode by collection of a specimen for one or more services (in circumstances other than those described in item 73922, 73923, 73924, 73925, 73926, 73927 or 73935) if the specimen is collected from a person in an institution by: (a) an approved pathology practitioner; or (b) an employee of an approved pathology authority | 17.60 |
73935 | Initiation of a patient episode by collection of a specimen for one or more services (in circumstances other than those described in item 73922, 73923, 73924, 73925, 73926 or 73927) if the specimen is collected from a person in an institution by: (a) an approved pathology practitioner of a prescribed laboratory; or (b) an employee of an approved pathology authority that operates a prescribed laboratory | 2.40 |
73936 | Initiation of a patient episode by collection of a specimen for one or more services (in circumstances other than those described in item 73922, 73923, 73924, 73925, 73926, 73927 or 73937) if the specimen is collected from the person by the person | 5.95 |
73937 | Initiation of a patient episode by collection of a specimen for one or more services (in circumstances other than those described in item 73922, 73923, 73924, 73925, 73926 or 73927) if the specimen is collected from the person by the person, and: (a) the service is performed in a prescribed laboratory; or (b) the person is a private patient in a recognised hospital | 2.40 |
73938 | Initiation of a patient episode by collection of a specimen for one or more services (in circumstances other than those described in item 73922, 73923, 73924, 73925, 73926, 73927 or 73939) if the specimen is collected by, or on behalf of, the treating practitioner | 7.95 |
73939 | Initiation of a patient episode by collection of a specimen for one or more services (in circumstances other than those described in item 73922, 73923, 73924, 73925, 73926 or 73927) if the specimen is collected by, or on behalf of, the treating practitioner and: (a) the service is performed in a prescribed laboratory; or (b) the person is a private patient in a recognised hospital | 2.40 |
Division 2.11—Group P11: specimen referred
2.11.1 Items in Group P11 not to apply in certain circumstances
(1) An item in Group P11 (Specimen referred) does not apply to a pathology service to which subsection 16A(7) of the Act applies.
(2) An item in Group P11 does not apply to a pathology service unless at least one item in Groups P1 to P8 also applies to the service.
(3) An item in Group P11 applies only to the approved pathology practitioner or approved pathology authority to whom the specimen mentioned in the item was referred.
(4) The fee mentioned in item 73940 applies only once for a single patient episode.
2.11.2 Application of an item in Group P11 to a service excludes certain other items
If item 73940 applies to a patient episode, none of the items in Group P10 apply to any pathology service rendered by the approved pathology authority or approved pathology practitioner who claimed item 73940 for the patient episode.
2.11.3 Circumstances in which an item in Group P11 does not apply
(1) An item in Group P11 does not apply to a referral if:
(a) a service for the same patient episode has been carried out by the referring approved pathology authority; and
(b) the approved pathology authority to which the referral is made is related to the referring approved pathology authority.
(2) An approved pathology authority is related to another approved pathology authority for subclause (1) if:
(a) both approved pathology authorities are employed (including employed under contract) by the same person, whether or not the person is also an approved pathology authority; or
(b) either of the approved pathology authorities is employed (including employed under contract) by the other; or
(c) both approved pathology authorities are corporations and are connected entities within the meaning of the Corporations Act 2001; or
(d) the approved pathology authorities are partners (whether or not either or both of the approved pathology authorities are individuals and whether or not other persons are in partnership with either or both of the approved pathology authorities); or
(e) both approved pathology authorities are operated by the Commonwealth or an authority of the Commonwealth; or
(f) both approved pathology authorities are operated by the same State or internal Territory or an authority of the same State or internal Territory.
(3) An item in Group P11 does not apply to a referral if the following common tests are referred either singly or in combination (except if the following items are mentioned in combination with other items not similarly specified): 65060, 65070, 65120, 66500, 66503, 66506, 66509, 66512, 66536, 66596, 69300, 69303, 69333 or 73527.
Group P11—Specimen referred |
Item | Pathology service | Fee ($) |
73940 | Receipt of a specimen by an approved pathology practitioner of an approved pathology authority from another approved pathology practitioner of another approved pathology authority | 10.25 |
Division 2.12—Group P12: management of bulk‑billed services
2.12.1 Application of items 74990 and 74991
(1) Despite clause 1.2.1:
(a) if the pathology service described in item 74991 is provided to a person, either that item or item 74990, but not both those items, applies to the service; and
(b) if item 74990 or 74991 applies to a pathology service, the fee specified in that item applies in addition to the fee specified in any other item in this table that applies to the service.
(2) For items 74990 and 74991:
bulk‑billed, for a pathology service, means:
(a) a medicare benefit is payable to a person for the service; and
(b) under an agreement entered into under section 20A of the Act:
(i) the person assigns to the practitioner by whom, or on whose behalf, the service is provided, his or her right to the payment of the medicare benefit; and
(ii) the practitioner accepts the assignment in full payment of his or her fee for the service provided.
Commonwealth concession card holder means a person who is a concessional beneficiary within the meaning given by subsection 84(1) of the National Health Act 1953.
unreferred service means a pathology service that:
(a) is provided to a person by, or on behalf of, a medical practitioner, being a medical practitioner who is not a consultant physician, or specialist, in any speciality (other than a medical practitioner who is, for the purposes of the Act, both a general practitioner and a consultant physician, or specialist, in a particular speciality); and
(b) has not been referred to the medical practitioner by another medical practitioner or person with referring rights.
(3) For item 74991:
ASGC means the document titled Australian Standard Geographical Classification (ASGC) (ABS catalogue number 1216.0), published by the Australian Bureau of Statistics in July 2010.
practice location, for the provision of a pathology service, means the place of practice for which the practitioner by whom, or on whose behalf, the service is provided, has been allocated a provider number by the Chief Executive Medicare.
regional, rural or remote area means an area classified as RRMAs 3‑7 under the Rural, Remote and Metropolitan Areas Classification.
Rural, Remote and Metropolitan Areas Classification has the meaning given by the general medical services table.
SLA means a Statistical Local Area specified in the ASGC.
SSD means a Statistical Subdivision specified in the ASGC.
Group P12—Management of bulk‑billed services |
Item | Pathology service | Fee ($) |
74990 | A pathology service to which an item in this table (other than this item or item 74991) applies if: (a) the service is an unreferred service; and (b) the service is provided to a person who is under 16 years or is a Commonwealth concession card holder; and (c) the person is not an admitted patient of a hospital; and (d) the service is bulk‑billed in respect of the fees for: (i) this item; and (ii) the other item in this table applying to the service | 7.05 |
74991 | A pathology service to which an item in this table (other than this item or item 74990) applies if: (a) the service is an unreferred service; and (b) the service is provided to a person who is under 16 years or is a Commonwealth concession card holder; and (c) the person is not an admitted patient of a hospital; and (d) the service is bulk‑billed in respect of the fees for: (i) this item; and (ii) the other item in this table applying to the service; and (e) the service is provided at, or from, a practice location in: (i) a regional, rural or remote area; or (ii) Tasmania; or (iii) a geographical area included in any of the following SSD spatial units: (A) Beaudesert Shire Part A; (B) Belconnen; (C) Darwin City; (D) Eastern Outer Melbourne; (E) East Metropolitan Perth; (F) Frankston City; (G) Gosford‑Wyong; (H) Greater Geelong City Part A; (I) Gungahlin‑Hall; (J) Ipswich City (Part in BSD); (K) Litchfield Shire; (L) Melton‑Wyndham; (M) Mornington Peninsula Shire; (N) Newcastle; (O) North Canberra; (P) Palmerston‑East Arm; (Q) Pine Rivers Shire; (R) Queanbeyan; (S) South Canberra; (T) South Eastern Outer Melbourne; | 10.65 |
| (U) Southern Adelaide; (V) South West Metropolitan Perth; (W) Thuringowa City Part A; (X) Townsville City Part A; (Y) Tuggeranong; (Z) Weston Creek‑Stromlo; (ZA) Woden Valley; (ZB) Yarra Ranges Shire Part A; or (iv) the geographical area included in the SLA spatial unit of Palm Island (AC) | |
Division 2.13—Group P13: bulk billing incentive for episodes consisting of a P10 service
Note: The payments mentioned in column 3 of Group P13 are additional payments for bulk billing a patient episode consisting of a pathology service to which a Group P10 item described in column 2 applies.
Group P13—Bulk billing incentive for episodes consisting of a P10 service |
Item | Pathology service | Fee ($) |
74992 | A patient episode that: (a) consists of a pathology service to which item 73920 applies; and (b) is bulk billed | 1.60 |
74993 | A patient episode that: (a) consists of a pathology service to which item 73922 or 73926 applies; and (b) is bulk billed | 3.75 |
74994 | A patient episode that: (a) consists of a pathology service to which item 73924 applies; and (b) is bulk billed | 3.25 |
74995 | A patient episode that: (a) consists of a pathology service to which item 73928, 73930 or 73936 applies; and (b) is bulk billed | 4.00 |
74996 | A patient episode that: (a) consists of a pathology service to which item 73932 or 73940 applies; and (b) is bulk billed | 3.70 |
74997 | A patient episode that: (a) consists of a pathology service item 73934 applies; and (b) is bulk billed | 3.30 |
74998 | A patient episode that: (a) consists of a pathology service to which item 73938 applies; and (b) is bulk billed | 2.00 |
74999 | A patient episode that: (a) consists of a pathology service to which item 73923, 73925, 73927, 73929, 73931, 73933, 73935, 73937 or 73939 applies; and (b) is bulk billed | 1.60 |
Part 3—Abbreviations
Note: A service or drug that is not mentioned in this Part must be written in full.
Abbreviations |
Test | Abbreviation | Item |
Abnormal haemoglobins | AH | 65117 |
Acetoacetate | ACAT | 66500 |
Acetylcholine receptor—tissue antigens—antibodies | ARA | 71165 |
Acid phosphatase | ACP | 66500 |
Actinomycetes—microbial antibody testing | ACT | 69384 |
Activated protein C resistance | APC | 65142, 65171, 65175–65179 |
Adenovirus—microbial antibody testing | ADE | 69384 |
Adrenal cortex—tissue antigens—antibodies | ADR | 71165 |
Adrenocorticotrophic hormone (ACTH) | ACTH | 66695 |
AFB microscopy and culture of sputum—one specimen | AFB1 | 69324 |
AFB microscopy and culture of sputum—2 specimens | AFB2 | 69327 |
AFB microscopy and culture of sputum—3 specimens | AFB3 | 69330 |
Alanine aminotransferase | ALT | 66500 |
Albumin | ALB | 66500 |
Alcohol (ethanol) | ETOH | 66626, 66800 |
Aldosterone | ALDS | 66695 |
Alkaline phosphatase | ALP | 66500 |
Alkaline phosphatase—isoenzymes | ALPI | 66641 |
Alpha‑1‑antitrypsin | AAT | 66635 |
Alpha‑fetoprotein | AFP | 66650–66653, 66743, 66750, 66751 |
Aluminium | AL | 66825, 66828 |
Aluminium—renal dialysis | ALR | 66671 |
Amikacin | | 66800 |
Amino acids | AA | 66752 |
Amiodarone | AMIO | 66812 |
Amitriptyline | AMIT | 66812 |
Ammonia | NH3 | 66500 |
Amniotic fluid examination | AFE | 66749 |
Amylase | AMS | 66500 |
Amylobarbitone | AMYL | 66812 |
Androstenedione | ANDR | 66695 |
Angiotensin converting enzyme | ACE | 66758 |
Antibiotic and antimicrobial chemotherapeutic agents—quantitation | QAA | 66800, 66812 |
Antibodies to extractable nuclear antigens—characterisation of antibodies if positive ENA | ENAP | 71103 |
Antibodies to extractable nuclear antigens—detection | ENA | 71101 |
Antibodies to nuclear antigens—detection | ANA | 71097 |
Antibodies to nuclear antigens—quantitation and measurement of DNA binding if positive ANA | ANAP | 71099 |
Antibodies to tissue antigens—acetylcholine receptor | ARA | 71165 |
Antibodies to tissue antigens—adrenal cortex | ADR | 71165 |
Antibodies to tissue antigens—ANCA‑myeloperoxidase | MPO | 71153 |
Antibodies to tissue antigens—ANCA‑PR3 | PR3 | 71153 |
Antibodies to tissue antigens—anti‑actin | AACT | 71119 |
Antibodies to tissue antigens—anti‑endomysial | EMA | 71163 |
Antibodies to tissue antigens—cardiolipin | ACL | 71165 |
Antibodies to tissue antigens—coeliac disease panel | CLC | 71163, 71164 |
Antibodies to tissue antigens—gastric parietal cell | PCA | 71119 |
Antibodies to tissue antigens—gliadin IgA | GLIA | 71163 |
Antibodies to tissue antigens—gliadin IgG | GLIG | 71163 |
Antibodies to tissue antigens—glomerular basement membrane | GBM | 71153 |
Antibodies to tissue antigens—heart | AHE | 71165 |
Antibodies to tissue antigens—histone | AHI | 71165 |
Antibodies to tissue antigens—insulin receptor antibodies | INSA | 71165 |
Antibodies to tissue antigens—intercellular cement substance of skin | ICCS | 71165 |
Antibodies to tissue antigens—intrinsic factor | AIF | 71165 |
Antibodies to tissue antigens—islet cell | AIC | 71165 |
Antibodies to tissue antigens—Jo‑1 | JO1 | 71119 |
Antibodies to tissue antigens—keratin | KERA | 71119 |
Antibodies to tissue antigens—liver/kidney microsomes | LKA | 71119 |
Antibodies to tissue antigens—lymphocyte | ALY | 71165 |
Antibodies to tissue antigens—mitochondria | MA | 71119 |
Antibodies to tissue antigens—neuron | ANE | 71165 |
Antibodies to tissue antigens—neutrophil cytoplasm | ANCA | 71153 |
Antibodies to tissue antigens—ovary | AOV | 71165 |
Antibodies to tissue antigens—parathyroid | PTHA | 71165 |
Antibodies to tissue antigens—platelet | APA | 71165 |
Antibodies to tissue antigens—PM‑Sc1 | PM1 | 71119 |
Antibodies to tissue antigens—reticulin | RCA | 71119 |
Antibodies to tissue antigens—salivary gland | ASG | 71165 |
Antibodies to tissue antigens—Scl‑70 | SCL | 71119 |
Antibodies to tissue antigens—skeletal muscle | SLA | 71165 |
Antibodies to tissue antigens—skin basement membrane | SKA | 71165 |
Antibodies to tissue antigens—smooth muscle | SMA | 71119 |
Antibodies to tissue antigens—thyroglobulin | ATG | 71165 |
Antibodies to tissue antigens—thyroid microsome | TMA | 71165 |
Antibodies to tissue antigens—tissue transglutaminase | TTG | 71163 |
Antibodies to tissue antigens—TSH receptor antibody test | TSHA | 71165 |
Antibody testing, microbial (see Microbial) | | |
Antigen testing, microbial (see Microbial) | | |
Antithrombin III | ATH | 65142, 65171, 65175–65179 |
Anti‑Xa activity | QAXA | 65147 |
Anus—cytology on specimens from | SMCY | 73043 |
Apolipoprotein B/A1 ratio | APO | 66536 |
Arsenic | AS | 66825, 66828 |
Aspartate aminotransferase | AST | 66500 |
Aspergillus—microbial antibody testing | ASP | 69384 |
Avian precipitins (bird fancier’s disease)—microbial antibody testing | APP | 69384 |
B12 vitamin | B12 | 66838 |
Barbiturate | BARB | 66800, 66812 |
Beryllium | BE | 66825, 66828 |
Beta‑2‑microglobulin | BMIC | 66629 |
Beta‑hydroxybutyrate | BHYB | 66500 |
Bicarbonate | HCO3 | 66500 |
Bilirubin (all fractions) | BILI | 66500 |
Bilirubin (all fractions)—in urine | UBIL | 66500 |
Bilirubin (all fractions)—neonatal | BILN | 66749 |
Bird fancier’s disease (see avian precipitins) | APP | 69384 |
Blastomyces—microbial antibody testing | BLM | 69384 |
Blood—compatibility testing | XMAT | 65099–65108 |
Blood—culture | BC | 69354–69360 |
Blood—faecal occult | FOB | 66764–66770 |
Blood—film | BF | 65066 |
Blood—full examination | FBE | 65070 |
Blood—gases | GAS | 66566 |
Blood—group and blood group antibodies | BGAB | 65096 |
Blood—group antibodies | BGA | 65111 |
Blood—group systems | BGS | 65093 |
Blood—grouping—ABO and RH (D antigen) | BG | 65090 |
Blood—viscosity | VISC | 65060 |
Body cavities—aspirations of—microscopy and culture of material from | MCPO | 69321 |
Body fluids—cytology | BFCY | 73045 |
Bone—low mineral densities | CBLB | 66773 |
Bone—metabolic bone disease | CBMB | 66776 |
Bone marrow examination—aspirate | BMEA | 65087 |
Bone marrow examination—trephine | BMET | 65084 |
Bordetella pertussis—microbial antibody testing | BOR | 69384 |
Borrelia burgdorferi—microbial antibody testing | BOB | 69384 |
Breath hydrogen test | BHT | 66674 |
Bromide | BRMD | 66812 |
Brucella—microbial antibody testing | BRU | 69384 |
C‑l esterase inhibitor—functional | CEIF | 66647 |
C‑l esterase inhibitor—quantitation | CEIQ | 66644 |
CA‑15.3 antigen | CA15 | 66650 |
CA‑19.9 antigen | CA19 | 66650 |
CA‑125 antigen | C125 | 66650 |
Cadmium | CD | 66825, 66828 |
Caeruloplasmin | CPLS | 66632 |
Calcitonin | CALT | 66695 |
Calcium—ionised | ICA | 66584 |
Calcium (total or corrected for albumin) | CA | 66500 |
Calculus analysis | CALC | 66590 |
Campylobacter jejuni—microbial antibody testing | CAM | 69384 |
Candida—microbial antibody testing | CAN | 69384 |
Carbamazepine (Tegretol) | CARB | 66800 |
Carboxyhaemoglobin | COHB | 65117 |
Carcinoembryonic antigen | CEA | 66650 |
Cardiac enzymes | CE | 66506 |
Cardiolipin—tissue antigens—antibodies | ACL | 71165 |
Catecholamines | CAT | 66779 |
Cell‑mediated immunity—delayed type—hypersensitivity test | CMI | 71137 |
Cell‑mediated immunity in blood | CMIB | 69471 |
Cervix—cytology—abnormalities | CCRA | 73055 |
Cervix—cytology—routine | CCR | 73053 |
Cervix—microscopy and culture of material from | MCGR | 69312 |
Characterisation of antibodies if positive ENA | ENAP | 71103 |
Chemicals, toxic (ingested or absorbed)—assays | DRGO | 66623 |
Chlamydia—investigation by any method | CHLM | 69494 |
Chlamydia—investigation by any method and N gonorrhoea by NAA methods | CHGO | 69494 |
Chlamydia—microbial antibody testing | CHL | 69384 |
Chlamydia—microbial antigen testing | MCCH | 69494 |
Chloral hydrate | CHHY | 66812 |
Chlorazepate | CHZP | 66812 |
Chloride | CL | 66500 |
Chloroquine | CLOQ | 66812 |
Chlorpromazine | CHLO | 66812 |
Cholesterol | CHOL | 66500 |
Cholesterol—HDL | HDLC | 66536 |
Cholinesterase | CHSE | 66758 |
Chorionic gonadotrophin—detection for pregnancy diagnosis | HCGP | 73527–73529 |
Chorionic gonadotrophin—quantitation | HCG | 66650–66653, 66750, 66751, 73529 |
Chromium | CR | 66825, 66828 |
Chromosome identification and banding | | 73287–73289 |
Chromosome identification—studies—blood | CSB | 73289 |
Chromosome identification—studies—other than blood | CS | 73287 |
Cimetidine | CMTD | 66812 |
Clobazam | CLOB | 66812 |
Clomipramine | CLOM | 66812 |
Clonazepam (Rivotril) | CLON | 66812 |
Clostridium difficile—microbial antigen testing | CLDT | 69363 |
Coagulation factor inhibitors by Bethesda assay | BETH | 65159 |
Coagulation—factors (see individual factors) | | |
Coagulation—studies | COAG | 65120 |
Coccidioides—microbial antibody testing | CCC | 69384 |
Coeliac antibodies | CLC | 71163, 71164 |
Cold agglutinins | CAG | 65114 |
Collagen—low bone | CBLB | 66773 |
Collagen—metabolic disease | CBMB | 66776 |
Compatibility testing | XMAT | 65099–65108 |
Complement, total haemolytic | COM | 71081 |
Complement, total haemolytic—components C3 | C3 | 71083 |
Complement, total haemolytic—components C4 | C4 | 71083 |
Complement, total haemolytic—other components | COMP | 71089 |
Complement, total haemolytic—properdin factor B | PFB | 71083 |
Copper | CU | 66819, 66822 |
Cortisol | CORT | 66695 |
Cortisol in saliva | CORS | 66711, 66712 |
Coxsackie B1‑6—microbial antibody testing | COX | 69384 |
C‑Peptide | CPEP | 66695 |
C‑reactive protein | CRP | 66500 |
Creatine kinase | CK | 66500 |
Creatine kinase—isoenzymes | CKI | 66518 |
Creatine kinase—isoenzymes (electrophoresis) | CKIE | 66518 |
Creatinine | C | 66500 |
Cryofibrinogen—detection and quantitation | CFID | 71064 |
Cryoglobulins—characterisation by electrophoresis and immunoelectrophoresis or immunofixation or isoelectric focusing | RYO | 71059 |
Cryoglobulins—detection and quantitation | CGLD | 71064 |
Cryptococcal antigen—microbial antigen testing | CRYN | 69494 |
Cryptococcus—microbial antibody testing | CRY | 69384 |
CSF antigens—group B streptococcus | STB | 69494 |
CSF antigens—Haemophilus influenzae | HI | 69494 |
CSF antigens—Neisseria meningitidis | NMG | 69494 |
CSF antigens—Streptococcus pneumoniae | SPN | 69494 |
CSF—microscopy and culture of material from | MCPO | 69321 |
Cultural examination of faeces | FCS | 69345 |
Cyclic AMP | CAMP | 66695 |
Cyclosporin A | CLSA | 66812 |
Cystine—qualitative | UCYS | 66752 |
Cystine—quantitative | CYST | 66752 |
Cytology—fine needle aspiration of solid tissues | FNCY | 73049 |
Cytology—fine needle aspiration of solid tissues—aspiration or attendance by a pathologist | FNCP | 73051 |
Cytology—from 3 sputum or urine specimens | SPCY | 73047 |
Cytology—from body fluids, sputum (one specimen), urine, washings or brushings | BFCY | 73045 |
Cytology—from cervix—abnormalities | CCRA | 73055 |
Cytology—from cervix—routine | CCR | 73053 |
Cytology—from skin, nipple discharge, lip, mouth, nose or anus | SMCY | 73043 |
Cytology—from vagina | CVO | 73057 |
Cytomegalovirus—microbial antibody testing | CMV | 69384 |
Cytomegalovirus serology in pregnancy—microbial antibody testing | CMVP | 69405–69411 |
D‑dimer test | DD | 65120 |
D vitamin | VITD | 66833 |
Dehydroepiandrosterone sulphate (DHEAS) | DHEA | 66695 |
Dengue—microbial antibody testing | DEN | 69384 |
11‑Deoxycortisol | DCOR | 66695 |
Desipramine | DESI | 66812 |
Dexamethasone | DXST | 66686 |
Dexamethasone—suppression test | DEXA | 66686 |
DHEAS (Dehydroepiandrosterone sulphate) | DHEA | 66695 |
Diazepam | DIAZ | 66812 |
Differential cell count | DIFF | 65070 |
Digoxin | DIG | 66800 |
Dihydrotestosterone | DHTS | 66695 |
Diphenylhydantoin (Dilantin) | DIL | 66812 |
Diphtheria—microbial antibody testing | DIP | 69384 |
Direct Coombs test | CMBS | 65114 |
Disopyramide (Rythmodan) | DISO | 66800 |
DNA binding—quantitation and measurement if positive ANA | ANAP | 71099 |
DNA (double‑stranded DNA) antibody | DSDNA | 71099 |
Donath Landsteiner antibody test | DLAT | 65075 |
Down’s syndrome and neural tube defects | NTDD | 66750, 66751 |
Doxepin hydrochloride | DOXE | 66812 |
Drugs—abuse treatment programme—assay | DATP | 66626 |
Drugs—inappropriate dosage—assay | DRGO | 66623 |
Drugs—therapeutic—assay (see individual drugs) | | 66800, 66812 |
Dynamic function tests | GHSE | 66686 |
Ear—microscopy and culture of material from | MCSW | 69303 |
Echinococcus—microbial antibody testing | ECC | 69384 |
Echis test | ECHI | 65120 |
ECHO‑coxsackie group—microbial antibody testing | ECH | 69384 |
Electrolytes | E | 66509 |
Electron microscopy of biopsy material | EM | 72851, 72852 |
Electrophoresis, and immunofixation or immunoelectrophoresis or isoelectric focusing—characterisation of cryoglobulins | RYO | 71059 |
Electrophoresis, and immunofixation or immunoelectrophoresis or isoelectric focusing—characterisation of paraprotein | PRYO | 71059 |
Electrophoresis, to demonstrate—creatine kinase isoenzymes | CKIE | 66518 |
Electrophoresis, to demonstrate—lactate dehydrogenase isoenzymes | LDI | 66641 |
Electrophoresis, to demonstrate—lipoprotein subclasses | LEPG | 66539 |
Electrophoresis, to demonstrate—quantitation of protein classes, or paraprotein | EPPI | 71057, 71058 |
Elements (see individual elements) | | |
Endomysium antibodies | EMA | 71163 |
Entamoeba histolytica—microbial antibody testing | AMO | 69384 |
Enzyme assays of solid tissue or tissues | ENZS | 66683 |
Enzyme histochemistry of skeletal muscle | EHSK | 72844 |
Eosinophil cationic protein | ECP | 71095 |
Epstein Barr virus—microbial antibody testing | EBV | 69472–69474 |
Erythrocyte—assessment of haemolysis | ERYH | 65075 |
Erythrocyte—assessment of metabolic enzymes | ERYM | 65075 |
Erythrocyte—count | RCC | 65070 |
Erythrocyte—sedimentation rate | ESR | 65060 |
Ethanol (alcohol) | ETOH | 66626, 66800 |
Ethosuximide (Zarontin) | ETHO | 66800 |
Extractable nuclear antigens—detection of antibodies to | ENA | 71101 |
Eye—microscopy and culture of material from | MCSW | 69303 |
Factor II | FII | 65150 |
Factor V | FV | 65150 |
Factor V Leiden mutation | FVLM | 73308, 73311 |
Factor VII | FVII | 65150 |
Factor VIII | VIII | 65150 |
Factor IX | FIX | 65150 |
Factor X | FX | 65150 |
Factor XI | FXI | 65150 |
Factor XII | FXII | 65150 |
Factor XIII | XIII | 65150 |
Factor XIII deficiency test | F13D | 65120 |
Faecal antigen test for Helicobacter pylori | FAHP | 69494 |
Faecal blood | FOB | 66764–66770 |
Faecal fat | FFAT | 66674 |
Faecal fat—haemoglobin | FFH | 66764 |
Faecal fat—reducing substances | FRS | 66761 |
Faeces—culture | FCS | 69345 |
Faeces—microscopy for parasites | OCP | 69336–69339 |
Ferritin (see also Iron studies) | FERR | 66593 |
Fibrin monomer | FM | 65120 |
Fibrinogen | FIB | 65120 |
Fibrinogen—degradation products | FDP | 65120 |
Fitzgerald factor | FGF | 65150 |
Flecainide | FLEC | 66812 |
Fletcher factor | FF | 65150 |
Fluorescent treponemal antibody—absorption test (FTA‑ABS)—microbial antibody testing | FTA | 69384 |
Fluoxetine | FLUX | 66812 |
Foetal red blood cells—Kliehauer | KLEI | 65162 |
Folate—serum | SF | 66840 |
Follicle stimulating hormone (FSH) | FSH | 66695 |
Fragile X | FXS | 73300, 73305 |
Frozen section diagnosis of biopsy material | FS | 72855, 72856 |
Fructosamine | FRUC | 66557 |
Full blood examination | FBE | 65070 |
Gamma glutamyl transpeptidase | GGT | 66500 |
Gastric parietal cell—tissue antigens—antibodies | PCA | 71119 |
Gastrin | GAST | 66695 |
Gentamicin | | 66800 |
Gliadin IgA—tissue antigens—antibodies | GLIA | 71163 |
Globulin | GLOB | 66500 |
Glomerular basement membrane—tissue antigens—antibodies | GBA | 71165 |
Glucagon | GLGO | 66695 |
Glucose | GLUC | 66500 |
Glucose—tolerance test | GTT | 66542 |
Glycosylated haemoglobin (Hb Alc) | GHB | 66551 |
Gold | AU | 66825, 66828 |
Group B streptococcus—CSF antigens | STB | 69494 |
Group B streptococcus—microbial antigen testing | STB | 69494 |
Group P9—simple basic pathology tests | | 73801–73811 |
Growth hormone | GH | 66695 |
Growth hormone—stimulation by exercise or L‑dopa | GHSE | 66686 |
Growth hormone—suppression by dexamethasone or glucose | GHSG | 66686 |
Haematocrit | HCT | 65070 |
Haemochromatosis | FEUP | 73317 |
Haemoglobin | HB | 65060 |
Haemoglobinopathy tests | HMGP | 65081 |
Haemophilus influenzae—CSF antigens | HI | 69494 |
Haemophilus influenzae—microbial antibody testing | HUS | 69384 |
Haemophilus influenzae—microbial antigen testing | HI | 69494 |
Haloperidol | HALO | 66812 |
Haptoglobins | HGLB | 66632 |
HDL cholesterol | HDLC | 66536 |
Heart—tissue antigens—antibodies | AHE | 71165 |
Heparin—test | HEPR | 65144 |
Hepatitis B or C confirmatory test | HSVP | 69484 |
Hepatitis C—detection | RNAC | 69499 |
Hepatitis C—genotype | GHCV | 69491 |
Hepatitis C—quantitation | THCV | 69488 |
Hepatitis investigation—3 markers | HEP3 | 69481 |
Hepatitis serology—in pregnancy | HEPP | 69405–69413 |
Hepatitis status or carriage—one marker | HEP1 | 69475 |
Hepatitis status or carriage—2 markers | HEP2 | 69478 |
Hepatitis status or carriage—3 markers | HEP3 | 69481 |
Herpes simplex virus—direct detection from clinical material | HSV | 69494 |
Herpes simplex virus—investigation by culture | HSVC | 69494 |
Herpes simplex virus—microbial antibody testing | HPA | 69384 |
Herpes simplex virus—microbial antigen testing | HSV | 69494 |
Heterophil antibodies | IM | 65114 |
HIAA (hydroxyindoleacetic acid) | HIAA | 66779 |
Histamine | HIAM | 66779 |
Histone—tissue antigens—antibodies | AHI | 71165 |
Histopathology of biopsy material | HIST | 72813–72836 |
Histoplasma—microbial antibody testing | HIP | 69384 |
HIV—antiretroviral therapy | TVLT | 69381 |
HIV—cerebrospinal fluid | CVLT | 69382 |
HIV—monitoring | MVLT | 69378 |
HLA typing—HLA class 1 | HLA1 | 71149 |
HLA typing—HLA class 2 | HLA2 | 71151 |
HLA typing—HLA‑B27 | HLAB | 71147 |
HMMA (hydroxy‑3‑methoxymandelic acid, previously known as VMA) | HMMA | 66779 |
HMPG (hydroxy‑methoxy phenylethylene glycol) | HMPG | 66779 |
Homovanillic acid | HVA | 66779 |
Hormone receptor assay—breast | HRA | 66662 |
Hormone receptor assay—ovary | HRO | 66662 |
Hormones—11 deoxycortisol | DCOR | 66695 |
Hormones—adrenocorticotrophic hormone | ACTH | 66695 |
Hormones—aldosterone | ALDS | 66695 |
Hormones and hormone binding proteins (see individual hormones and proteins) | | 66695 |
Hormones—androstenedione | ANDR | 66695 |
Hormones—calcitonin | CALT | 66695 |
Hormones—cortisol | CORT | 66695 |
Hormones—C‑Peptide | CPEP | 66695 |
Hormones—cyclic AMP | CAMP | 66695 |
Hormones—dehydroepiandrosterone sulphate (DHEAS) | DHEA | 66695 |
Hormones—dihydrotestosterone | DHTS | 66695 |
Hormones—follicle stimulating hormone | FSH | 66695 |
Hormones—gastrin | GAST | 66695 |
Hormones—glucagon | GLGO | 66695 |
Hormones—growth hormone | GH | 66695 |
Hormones—growth hormone—stimulation by exercise or L‑dopa | GHSE | 66686 |
Hormones—growth hormone—suppression by dexamethasone or glucose | GHSG | 66686 |
Hormones—hormone receptor assay—breast | HRA | 66662 |
Hormones—hormone receptor assay—ovary | HRO | 66662 |
Hormones—human chorionic gonadotrophin—detection for pregnancy diagnosis | HCGP | 73527, 73529 |
Hormones—human chorionic gonadotrophin—quantitation | HCG | 66650–66653, 66750, 66751, 73529 |
Hormones—hydroxyprogesterone | OHP | 66695 |
Hormones—insulin | INS | 66695 |
Hormones—luteinizing hormone | LH | 66695 |
Hormones—oestradiol | E2 | 66695 |
Hormones—oestriol | E3 | 66750, 66751 |
Hormones—oestrone | E1 | 66695 |
Hormones—parathyroid hormone | PTH | 66695 |
Hormones—progesterone | PROG | 66695 |
Hormones—prolactin | PROL | 66695 |
Hormones—renin | REN | 66695 |
Hormones—sex hormone binding globulin | SHBG | 66695 |
Hormones—somatomedin | SOMA | 66695 |
Hormones—stimulation by exercise or L‑dopa | GHSE | 66686 |
Hormones—suppression by dexamethasone or glucose | GHSG | 66686 |
Hormones—testosterone | TES | 66695 |
Hormones—urine steroid fraction or fractions | USF | 66695 |
Hormones—vasoactive intestinal peptide | VIP | 66695 |
Hormones—vasopressin | ADH | 66695 |
Huhner’s test | HT | 73521 |
Human chorionic gonadotrophin—detection for pregnancy diagnosis | HCGP | 73527, 73529 |
Human chorionic gonadotrophin—quantitation | HCG | 66650–66653, 66750, 66751, 73529 |
Human papillomaviruses | HPV | 69418 |
HVA (homovanillic acid) | HVA | 66779 |
Hydatid—microbial antibody testing | HYD | 69384 |
Hydroxy‑3‑methoxymandelic acid (previously known as VMA) | HMMA | 66779 |
Hydroxychloroquine | HOCQ | 66812 |
Hydroxyindoleacetic acid | HIAA | 66779 |
Hydroxy methoxy phenylethylene glycol | HMPG | 66779 |
Hydroxyprogesterone | OHP | 66695 |
Hydroxyproline | HYDP | 66752 |
Imipramine | IMIP | 66812 |
Immediate frozen section diagnosis of biopsy material | FS | 72855–72856 |
Immunocyto. one‑3 antibodies | ICC | 73059, 73061 |
Immunocyto. 4+ antibodies | ICC1 | 73060 |
Immunoelectrophoresis and electrophoresis—characterisation of cryoglobulins | RYO | 71059 |
Immunoelectrophoresis and electrophoresis—characterisation of paraprotein | PPRO | 71059 |
Immunoglobulins—A | IGA | 71066 |
Immunoglobulins—D | IGD | 71074 |
Immunoglobulins—E (total) | IGE | 71075–71079 |
Immunoglobulins—G | IGG | 71068 |
Immunoglobulins—G, 4 subclasses | SIGG | 71073 |
Immunoglobulins—M | IGM | 71072 |
Immunohistochemical investigation of biopsy material | HIS | 72846–72848 |
Infectious mononucleosis | IM | 69384 |
Influenza A—microbial antibody testing | FLA | 69384 |
Influenza B—microbial antibody testing | FLB | 69384 |
Insulin | INS | 66695 |
Insulin receptor antibodies—tissue antigens—antibodies | INSA | 71165 |
Insulin—tissue antigens—antibodies | AINS | 71165 |
Intercellular cement substance of skin—tissue antigens—antibodies | ICCS | 71165 |
Intestinal disaccharidases | INTD | 66680 |
Intrinsic factor—tissue antigens—antibodies | AIF | 71165 |
Iron studies (iron, transferrin and ferritin) | IS | 66596 |
Islet cell—tissue antigens—antibodies | AIC | 71165 |
Isoelectric focusing and electrophoresis—characterisation of cryoglobulins | RYO | 71059 |
Isoelectric focusing and electrophoresis—characterisation of paraprotein | PPRO | 71059 |
Jo—1—tissue antigens—antibodies | JO1 | 71119 |
Keratin—tissue antigens—antibodies | KERA | 71119 |
Kleihauer test | KLEI | 65162 |
Lactate | LACT | 66500 |
Lactate—dehydrogenase | LDH | 66500 |
Lactate—dehydrogenase isoenzymes | LDI | 66641 |
Lamellar body phospholipid | LBPH | 66749 |
Lead | PB | 66665 |
Lecithin/sphingomyelin ratio (amniotic fluid) | LS | 66749 |
Legionella pneumophila—serogroup 1—microbial antibody testing | LP1 | 69384 |
Legionella pneumophila—serogroup 2—microbial antibody testing | LP2 | 69384 |
Leishmaniasis—microbial antibody testing | LEI | 69384 |
Leptospira—microbial antibody testing | LEP | 69384 |
Leucocyte count | WCC | 65070 |
Leucocyte count—3 surface markers—blood, CSF, serous fluid | LMH3 | 71139 |
Leucocyte count—3 surface markers—tissue | LMT3 | 71141 |
Leucocyte count—6 surface markers—blood, CSF, serous fluid and tissue(s) | LMHT | 71145 |
Leucocyte count—6 surface markers—blood, CSF, serous fluid or tissue | LM6 | 71143 |
Leucocyte count—CD34 surface marker only—blood | LMCD34 | 71146 |
Lignocaine | LIGN | 66800 |
Lip—cytology on specimens from | SMCY | 73043 |
Lipase | LIP | 66500 |
Lipid studies | FATS | 66500 |
Lipoprotein subclasses—electrophoresis | LEPG | 66539 |
Listeria—microbial antibody testing | LIS | 69384 |
Lithium | LI | 66800 |
Liver function tests | LFT | 66515 |
Liver/kidney microsomes—tissue antigens—antibodies | LKA | 71119 |
Lupus anticoagulant | LUPA | 65137, 65142, 65175–65179 |
Luteinizing hormone | LH | 66695 |
Lymphocyte—tissue antigens—antibodies | ALY | 71165 |
Lymphocytes—functional tests—one test | LF1 | 71127 |
Lymphocytes—functional tests—2 tests | LF2 | 71129 |
Lymphocytes—functional tests—3 tests | LF3 | 71131 |
Magnesium | MG | 66500 |
Mammary serum antigen | MSA | 66650 |
Manganese | MN | 66819, 66822 |
Mantoux test | MANT | 73811 |
Measles—microbial antibody testing | MEA | 69384 |
Mercury | HG | 66825, 66828 |
Metabolic bone disease | CBMB | 66776 |
Metanephrines | MNEP | 66779 |
Methadone | MTDN | 66812 |
Methaemalbumin detection (Schumm’s test) | SCHM | 65117 |
Methotrexate | MTTA | 66812 |
Methsuximide | MSUX | 66812 |
Methylphenobarbitone | MPBT | 66812 |
Metronidazole | MRDZ | 66812 |
Mexiletine (Mexitil) | MEX | 66812 |
Mianserin | MIAS | 66812 |
Microalbumin | MALB | 66560 |
Microbial antibody testing—actinomycetes | ACT | 69384 |
Microbial antibody testing—adenovirus | ADE | 69384 |
Microbial antibody testing—aspergillus | ASP | 69384 |
Microbial antibody testing—avian precipitins (bird fancier’s disease) | APP | 69384 |
Microbial antibody testing—Blastomyces | BLM | 69384 |
Microbial antibody testing—Bordetella pertussis | BOR | 69384 |
Microbial antibody testing—Borrelia burgdorferi | BOB | 69384 |
Microbial antibody testing—Brucella | BRU | 69384 |
Microbial antibody testing—Campylobacter jejuni | CAM | 69384 |
Microbial antibody testing—Candida | CAN | 69384 |
Microbial antibody testing—Chlamydia | CHL | 69384 |
Microbial antibody testing—Coccidioides | CCC | 69384 |
Microbial antibody testing—Coxsackie B1‑6 | COX | 69384 |
Microbial antibody testing—Cryptococcus | CRY | 69384 |
Microbial antibody testing—cytomegalovirus | CMV | 69384 |
Microbial antibody testing—cytomegalovirus serology in pregnancy | CMVP | 69384 |
Microbial antibody testing—dengue | DEN | 69384 |
Microbial antibody testing—diphtheria | DIP | 69384 |
Microbial antibody testing—echinococcus | ECC | 69384 |
Microbial antibody testing—echo‑coxsackie group | ECH | 69384 |
Microbial antibody testing—Entamoeba histolytica | AMO | 69384 |
Microbial antibody testing—Epstein Barr virus | EBV | 69472–69474 |
Microbial antibody testing—fluorescent treponemal antibody—absorption test (FTA‑ABS) | FTA | 69384 |
Microbial antibody testing—Haemophilus influenzae | HUS | 69384 |
Microbial antibody testing—herpes simplex virus | HPA | 69384 |
Microbial antibody testing—Histoplasma | HIP | 69384 |
Microbial antibody testing—Human Immunodeficiency Virus | | 69384 |
Microbial antibody testing—hydatid | HYD | 69384 |
Microbial antibody testing—infectious mononucleosis | IM | 69384 |
Microbial antibody testing—influenza A | FLA | 69384 |
Microbial antibody testing—influenza B | FLB | 69384 |
Microbial antibody testing—Legionella pneumophila—serogroup 1 | LP1 | 69384 |
Microbial antibody testing—Legionella pneumophila—serogroup 2 | LP2 | 69384 |
Microbial antibody testing—leishmaniasis | LEI | 69384 |
Microbial antibody testing—Leptospira | LEP | 69384 |
Microbial antibody testing—Listeria | LIS | 69384 |
Microbial antibody testing—measles | MEA | 69384 |
Microbial antibody testing—Micropolyspora faeni | MIC | 69384 |
Microbial antibody testing—mumps | MUM | 69384 |
Microbial antibody testing—Murray Valley encephalitis | MVE | 69384 |
Microbial antibody testing—Mycoplasma pneumoniae | MYC | 69384 |
Microbial antibody testing—Neisseria meningitidis | MEN | 69384 |
Microbial antibody testing—Newcastle disease | NCD | 69384 |
Microbial antibody testing—parainfluenza 1 | PF1 | 69384 |
Microbial antibody testing—parainfluenza 2 | PF2 | 69384 |
Microbial antibody testing—parainfluenza 3 | PF3 | 69384 |
Microbial antibody testing—paratyphi | PTY | 69384 |
Microbial antibody testing—pertussis | PER | 69384 |
Microbial antibody testing—poliomyelitis | PLO | 69384 |
Microbial antibody testing—Proteus OX 19 | POX | 69384 |
Microbial antibody testing—Proteus OXK | POK | 69384 |
Microbial antibody testing—Q fever | QFF | 69384 |
Microbial antibody testing—rapid plasma reagin test | RPR | 69384 |
Microbial antibody testing—respiratory syncytial virus | RSV | 69384 |
Microbial antibody testing—Ross River virus | RRV | 69384 |
Microbial antibody testing—rubella | RUB | 69384 |
Microbial antibody testing—Salmonella typhi (H) | SAH | 69384 |
Microbial antibody testing—Salmonella typhi (O) | SAO | 69384 |
Microbial antibody testing—Schistosoma | STO | 69384 |
Microbial antibody testing—streptococcal serology—anti‑DNASE B titre | ADNB | 69384 |
Microbial antibody testing—streptococcal serology—anti‑streptolysin O titre | ASOT | 69384 |
Microbial antibody testing—Streptococcus pneumoniae | PCC | 69384 |
Microbial antibody testing—tetanus | TET | 69384 |
Microbial antibody testing—Thermoactinomyces vulgaris | THE | 69384 |
Microbial antibody testing—thermopolyspora | TPS | 69384 |
Microbial antibody testing—Toxocara | TOC | 69384 |
Microbial antibody testing—Toxoplasma | TOX | 69384 |
Microbial antibody testing—TPHA (Treponema pallidum haemagglutination test) | TPHA | 69384 |
Microbial antibody testing—Treponema pallidum haemagglutination test | TPHA | 69384 |
Microbial antibody testing—trichinosis | TOS | 69384 |
Microbial antibody testing—typhus, Weil‑Felix | TYP | 69384 |
Microbial antibody testing—Varicella zoster | VCZ | 69384 |
Microbial antibody testing—VDRL (Venereal Disease Research Laboratory) | VDRL | 69384 |
Microbial antibody testing—Yersinia enterocolitica | YER | 69384 |
Microbial antigen testing—Chlamydia | MCCH | 69494 |
Microbial antigen testing—Clostridium difficile | CLDT | 69363 |
Microbial antigen testing—group B streptococcus | STB | 69494 |
Microbial antigen testing—Haemophilus influenzae | HI | 69494 |
Microbial antigen testing—herpes simplex virus | HSV | 69494 |
Microbial antigen testing—Neisseria gonorrhoeae | GON | 69494 |
Microbial antigen testing—Neisseria meningitidis | NMG | 69494 |
Microbial antigen testing—respiratory syncytial virus | RSVN | 69494 |
Microbial antigen testing—Streptococcus pneumonia | SPN | 69494 |
Microbial antigen testing—Varicella zoster | VCZN | 69494 |
Micropolyspora faeni | MIC | 69384 |
Microscopic examination of—faeces for parasites | OCP | 69336–36939 |
Microscopic examination of—wet film material other than blood | MWFM | 69300 |
Microscopy and culture of—material from nose, throat, eye or ear | MCSW | 69303 |
Microscopy and culture of—material from skin | MCSK | 69309 |
Microscopy and culture of—postoperative wounds, aspirates of body cavities | MCPO | 69321 |
Microscopy and culture of—specimens of sputum | MCSP | 69318 |
Microscopy and culture of—specimens of sputum, urine or other body fluids for mycobacteria—one specimen | AFB1 | 69324 |
Microscopy and culture of—specimens of sputum, urine or other body fluids for mycobacteria—2 specimens | AFB2 | 69327 |
Microscopy and culture of—specimens of sputum, urine or other body fluids for mycobacteria—3 specimens | AFB3 | 69330 |
Microscopy and culture of—superficial sites | MCSS | 69306 |
Microscopy and culture of—urethra, vagina, cervix or rectum | MCGR | 69312 |
Microscopy and culture to detect pathogenic micro‑organisms including Chlamydia | MCCH | 69494 |
Microscopy, culture, identification and sensitivity of urine | UMCS | 69333 |
Mitochondria—tissue antigens—antibodies | MA | 71119 |
Mouth—cytology on specimens from | SMCY | 73043 |
Mumps—microbial antibody testing | MUM | 69384 |
Murray Valley encephalitis—microbial antibody testing | MVE | 69384 |
Mycobacteria microscopy and culture of sputum—one specimen | AFB 1 | 69324 |
Mycobacteria microscopy and culture of sputum—2 specimens | AFB 2 | 69327 |
Mycobacteria microscopy and culture of sputum—3 specimens | AFB 3 | 69333 |
Mycoplasma pneumoniae—microbial antibody testing | MYC | 69384 |
Myoglobin | MYOG | 66518 |
N‑acetyl procainamide | NAPC | 66812 |
Neisseria gonorrhoeae by NAA techniques and chlamydia by any method | CHGO | 69494 |
Neisseria gonorrhoeae—microbial antigen testing | GON | 69494 |
Neisseria meningitidis—antigens | NMG | 69494 |
Neisseria meningitidis—microbial antibody testing | MEN | 69384 |
Neisseria meningitidis—microbial antigen testing | NMG | 69494 |
Netilmicin | | 66800 |
Neural tube defects and Down’s syndrome | NTDD | 66750, 66751 |
Neuron—tissue antigens—antibodies | ANE | 71165 |
Neutrophil cytoplasm—tissue antigens—antibodies | ANCA | 71165 |
Neutrophil functions | NFT | 71135 |
Newcastle disease—microbial antibody testing | NCD | 69384 |
Nickel | NI | 66825, 66828 |
Nipple discharge—cytology on specimens from | SMCY | 73043 |
Nitrazepam | NITR | 66812 |
Nordothiepin | NDIP | 66812 |
Norfluoxetine | NFLE | 66812 |
Nortriptyline | NORT | 66812 |
Nose—cytology on specimens from | SMCY | 73043 |
Nose—microscopy and culture of material from | MCSW | 69303 |
Nuclear antigens—detection of antibodies to | ANA | 71097 |
Oestradiol | E2 | 66695 |
Oestriol | E3 | 66750, 66751 |
Oestrone | E1 | 66695 |
Oligoclonal proteins | OGP | 71062 |
Op/biopsy specimens—microscopy and culture of material from | MCPO | 69321 |
Oral glucose challenge test—gestational diabetes | OGCT | 66545 |
Oral glucose tolerance test—gestational diabetes | GTTP | 66542 |
Osmolality, serum or urine | OSML | 66563 |
Ovary—tissue antigens—antibodies | AOV | 71165 |
Oxalate | OXAL | 66752 |
Oxazepam | OXAZ | 66812 |
PAA (phenyl acetic acid) | PAA | 66779 |
Palmitic acid in amniotic fluid | PALM | 66749 |
Pap smear | CCR | 73053 |
Papanicolaou test | CCR | 73053 |
Paracetamol | PARA | 66800 |
Parainfluenza 1—microbial antibody testing | PF1 | 69384 |
Parainfluenza 2—microbial antibody testing | PF2 | 69384 |
Parainfluenza 3—microbial antibody testing | PF3 | 69384 |
Paraprotein characterisation—by electrophoresis and immunoelectrophoresis or immunofixation or isoelectric focusing | PPRO | 71059 |
Paraprotein characterisation—on concurrently collected serum or urine | PPSU | 71060 |
Paraprotein quantitation—by electrophoresis | EPPI | 71057 |
Paraquat | PARQ | 66812 |
Parasites—microscopic examination of faeces | OCP | 69336–69339 |
Parathyroid hormone (PTH) | PTH | 66695 |
Parathyroid—tissue antigens—antibodies | PTHA | 71165 |
Paratyphi—microbial antibody testing | PTY | 69384 |
Partial thromboplastin time | PTT | 65120 |
Patient episode initiation fees | PEI | 73922–73939 |
Pentobarbitone | PENT | 66812 |
Perhexiline | PHEX | 66812 |
Pertussis—microbial antibody testing | PER | 69384 |
Phenobarbitone | PHBA | 66800 |
Phensuximide | PHEN | 66812 |
Phenylacetic acid | PAA | 66779 |
Phenytoin | PHEY | 66800 |
Phosphate | PHOS | 66500 |
Phosphatidylglycerol | PTGL | 66749 |
Platelet—aggregation | PLTG | 65144 |
Platelet—count | PLTC | 65070 |
Platelet—tissue antigens—antibodies | APA | 71165 |
PM‑Sc1—tissue antigens—antibodies | PM1 | 71119 |
Poliomyelitis—microbial antibody testing | PLO | 69384 |
Porphobilinogen in urine | UPG | 66782 |
Porphyrins in urine—qualitative test | UPR | 66782 |
Porphyrins—quantitative test, one or more fractions | PR | 66785 |
Potassium | K | 66500 |
Prealbumin | PALB | 66632 |
Prednisolone | PRED | 66812 |
Pregnancy serology—one test | MSP1 | 69405 |
Pregnancy serology—2 tests | MSP2 | 69408 |
Pregnancy serology—3 tests | MSP3 | 69411 |
Pregnancy serology—4 tests | MSP4 | 69413 |
Pregnancy testing | | 73806 |
Pregnancy testing—diagnosis of Down’s syndrome and neural tube defect | NTDD | 66750, 66751 |
Pregnancy testing—HCG detection | HCG | 73527, 73529 |
Pregnancy testing—HCG quantitation | HCG | 73529 |
Primidone | PRIM | 66800 |
Procainamide | PCAM | 66800 |
Progesterone | PROG | 66695 |
Prolactin | PROL | 66695 |
Propranolol | PPNO | 66812 |
Prostate specific antigen | PSA | 66655–66659 |
Protein—C | PROC | 65142, 65171, 65175–65179 |
Protein—S | PROS | 65142, 65171, 65175–65179 |
Protein, quantitation of—alpha fetoprotein | AFP | 66650–66653, 66743, 66750, 66751 |
Protein, quantitation of—alpha‑l‑antitrypsin | AAT | 66635 |
Protein, quantitation of—beta‑2‑microglobulin | BMIC | 66629 |
Protein, quantitation of—C‑l esterase inhibitor | CEI | 66644 |
Protein, quantitation of—caeruloplasmin | CPLS | 66632 |
Protein, quantitation of—classes or presence and amount of paraprotein by electrophoresis | EPPI | 71057, 71058 |
Protein, quantitation of—ferritin (see also Iron studies) | FERR | 66593 |
Protein, quantitation of—for Down’s syndrome and neural tube defect testing | NTDD | 66750, 66751 |
Protein, quantitation of—haptoglobins | HGLB | 66632 |
Protein, quantitation of—microalbumin | MALB | 66560 |
Protein, total—quantitation of | PROT | 66500 |
Proteus OX 19—microbial antibody testing | POX | 69384 |
Proteus OXK—microbial antibody testing | POK | 69384 |
Prothrombin gene mutation | PGM | 73308, 73311 |
Prothrombin time | PT | 65120 |
Pyruvate | PVTE | 66500 |
Q fever—microbial antibody testing | QFF | 69384 |
Quinalbarbitone | QUIB | 66812 |
Quinidine | QUIN | 66800 |
Quinine | QNN | 66812 |
Rapid plasma reagin test—microbial antibody testing | RPR | 69384 |
RAST | RAST | 71079 |
Rectum—microscopy and culture of material from | MCGR | 69312 |
Red blood cells—Kleihauer | KLEI | 65162 |
Red cell porphyrins—qualitative test | RCP | 66782 |
Referred specimen fee | | 73940 |
Renin | REN | 66695 |
Reptilase test | REPT | 65120 |
Respiratory syncytial virus—microbial antibody testing | RSV | 69384 |
Respiratory syncytial virus—microbial antigen testing | RSVN | 69494 |
Reticulin—tissue antigens—antibodies | RCA | 71119 |
Reticulocyte count | RETC | 65072 |
Rheumatoid factor | RF | 71106 |
Rheumatoid factor—quantitation | RFQ | 71106 |
Ross River virus—microbial antibody testing | RRV | 69384 |
RSV (respiratory syncytial virus)—microbial antibody testing | RSV | 69384 |
RSV (respiratory syncytial virus)—microbial antigen testing | RSVN | 69494 |
Rubella—serology | RUB | 69384 |
Salicylate (aspirin) | SALI | 66800 |
Salivary gland—tissue antigens—antibodies | ASG | 71165 |
Salmonella typhi (H)—microbial antibody testing | SAH | 69384 |
Salmonella typhi (O)—microbial antibody testing | SAO | 69384 |
Schistosoma—microbial antibody testing | STO | 69384 |
Scl‑70—tissue antigens—antibodies | SCL | 71119 |
Selenium | SE | 66819, 66822 |
Semen examination | SEE | 73523 |
Semen examination—for spermatozoa (post vasectomy) | SES | 73521 |
Serology—in pregnancy (see Pregnancy serology) | | |
Serotonin | 5HT | 66779 |
Serum—B12 | B12 | 66838 |
Serum—folate | SF | 66840 |
Sex hormone binding globulin | SHBG | 66695 |
Skeletal muscle—tissue antigens—antibodies | SLA | 71165 |
Skin—cytology | SMCY | 73043 |
Skin—microscopy and culture of material from | MCSS | 69306 |
Skin—microscopy, culture and Chlamydia of material from | MCSK | 69309 |
Skin basement membrane—tissue antigens—antibodies | SKA | 71165 |
Smooth muscle—tissue antigens—antibodies | SMA | 71119 |
Snake venom | HISS | 66623 |
Sodium | NA | 66500 |
Solid tissue or tissues—chemical assays | ENZS | 66683 |
Solid tissue or tissues—cytology of fine needle aspiration | FNCY | 73049 |
Solid tissue or tissues—cytology of fine needle aspiration by, or in presence of pathologist | FNCP | 73051 |
Somatomedin | SOMA | 66695 |
Sotalol | SALL | 66812 |
Specific IgE or IgG antibodies | RAST | 71079 |
Specimen referred fee | | 73940 |
Sperm antibodies | SAB | 73525 |
Sperm antibodies—penetrating ability | SPA | 73525 |
Sputum—cytology—one specimen | BFCY | 73045 |
Sputum—cytology—3 specimens | SPCY | 73047 |
Sputum—for mycobacteria—one specimen | AFB1 | 69324 |
Sputum—for mycobacteria—2 specimens | AFB2 | 69327 |
Sputum—for mycobacteria—3 specimens | AFB3 | 69330 |
Sputum—microscopy and culture of specimens | MCSP | 69318 |
Stelazine | STEL | 66812 |
Steroid fraction or fractions in urine | USF | 66695 |
Streptococcal serology—anti‑DNASE B titre—microbial antibody testing | ADNB | 69384 |
Streptococcal serology—anti‑streptolysin O titre—microbial antibody testing | ASOT | 69384 |
Streptococcus—Group B | STB | 69494 |
Streptococcus pneumoniae—CSF antigens | SPN | 69494 |
Streptococcus pneumoniae—microbial antibody testing | PCC | 69384 |
Streptococcus pneumoniae—microbial antigen testing | SPN | 69494 |
Strontium | SR | 66825, 66828 |
Stypven test | STYP | 65120 |
Sugar water test | SWT | 65075 |
Sulthiame (Ospolot) | SUL | 66812 |
Supplementary testing for Hepatitis C antibodies | HCST | 69441 |
Syphilis serology | STS | 69387 |
Testosterone | TES | 66695 |
Tetanus—microbial antibody testing | TET | 69384 |
Thalassaemia studies | TS | 65078 |
Theophylline | THEO | 66800 |
Thermoactinomyces vulgaris—microbial antibody testing | THE | 69384 |
Thermopolyspora—microbial antibody testing | TPS | 69384 |
Thiopentone | TOPO | 66812 |
Thioridazine | THIO | 66812 |
Throat—microscopy and culture of material from | MCSW | 69303 |
Thrombin time | TT | 65120 |
Thrombophilia testing (see individual thrombophilia tests) | | |
Thyroglobulin | TGL | 66650 |
Thyroglobulin—tissue antigens—antibodies | ATG | 71165 |
Thyroid function tests (including TSH) | TFT | 66719 |
Thyroid microsome—tissue antigens—antibodies | TMA | 71165 |
Thyroid stimulating hormone (if requested on its own, or as a preliminary test to thyroid function testing) | TSH | 66716 |
Thyroid stimulating hormone (if requested with other hormones referred to in item 66695) | TSH | 66722–66734 |
Tissue transglutaminase antibodies | TTG | 71163 |
Tobramycin | | 66800 |
Total protein | PROT | 66500 |
Toxocara—microbial antibody testing | TOC | 69384 |
Toxoplasma—microbial antibody testing | TOX | 69384 |
Treponema pallidum haemagglutination test—microbial antibody testing | TPHA | 69384 |
Trichinosis—microbial antibody testing | TOS | 69384 |
Triglycerides | TRIG | 66500 |
Trimipramine | TRIM | 66812 |
Troponin | TROP | 66518 |
Tryptic activity in faeces | TAF | 66677 |
TSH receptor antibody test—tissue antigens—antibodies | TSHA | 71165 |
Tuberculosis | MANT | 73811 |
Tumour markers—CA‑15.3 antigen | CA15 | 66650 |
Tumour markers—CA‑19.9 antigen | CA19 | 66650 |
Tumour markers—CA‑125 antigen | C125 | 66650 |
Tumour markers—carcinoembryonic antigen | CEA | 66650 |
Tumour markers—mammary serum antigen | MSA | 66650 |
Tumour markers—prostate specific antigen | PSA | 66656 |
Tumour markers—prostatic acid phosphatase—one or more fractions | ACP | 66656 |
Tumour markers—thyroglobulin | TGL | 66650 |
Typhus, Weil‑Felix—microbial antibody testing | TYP | 69384 |
Urate | URAT | 66500 |
Urea | U | 66500 |
Urea, electrolytes, creatinine | U&E | 66515 |
Urethra—microscopy and culture of material from | MCGR | 69312 |
Urine—acidification test | UAT | 66587 |
Urine—catalase test | UCAT | 73805 |
Urine—cystine (cysteine) | UCYS | 66782 |
Urine—cytology—on one specimen | BFCY | 73045 |
Urine—cytology—on 3 specimens | SPCY | 73047 |
Urine—haemoglobin | UHB | 66782 |
Urine—microscopy, culture, identification and sensitivity | UMCS | 69333 |
Urine—porphobilinogen | UPG | 66782 |
Urine—porphyrins—qualitative test | UPR | 66782 |
Urine—steroid fraction or fractions | USF | 66695 |
Urine—urobilinogen | UUB | 66782 |
Vagina—cytology on specimens from | CVO | 73057 |
Vagina—microscopy and culture of material from | MCGR | 69312 |
Valproate (Epilim) | VALP | 66800 |
Vancomycin | VAN | 66800 |
Varicella zoster—microbial antibody testing | VCZ | 69384 |
Varicella zoster—microbial antigen testing | VCZN | 69494 |
Vasoactive intestinal peptide | VIP | 66695 |
Vasopressin | ADH | 66695 |
VDRL (Venereal Disease Research Laboratory)—microbial antibody testing | VDRL | 69384 |
Viscosity of blood or plasma | VISC | 65060 |
Vitamins—B12 | B12 | 66838 |
Vitamins—D | VITD | 66833 |
Vitamins—folate | SF | 66840 |
Vitamins—quantitation of A, B1, B2, B3, B6, C or E | VIT | 66605 |
VMA (see HMMA) | | |
Von Willebrand’s factor | VWF | 65150 |
Von Willebrand’s factor antigen | VWA | 65150 |
Warfarin | WFR | 66812 |
Yersinia enterocolitica—microbial antibody testing | YER | 69384 |
Zinc | ZN | 66667–66670 |
Part 4—Complexity levels for histopathology items
Complexity levels for histopathology items |
Specimen type | Complexity level |
Adrenal resection, neoplasm | 5 |
Adrenal resection, not neoplasm | 4 |
Anus, all specimens not otherwise specified | 3 |
Anus, neoplasm, biopsy | 4 |
Anus, neoplasm, radical resection | 6 |
Anus, submucosal resection—neoplasm | 5 |
Appendix | 3 |
Artery, all specimens not otherwise specified | 3 |
Artery, biopsy | 4 |
Bartholin’s gland—cyst | 3 |
Bile duct, resection—all specimens | 6 |
Bone—all specimens not otherwise specified | 4 |
Bone, biopsy, curettings or fragments—lesion | 5 |
Bone, biopsy or curettings quantitation—metabolic disease | 6 |
Bone, femoral head | 4 |
Bone marrow, biopsy | 4 |
Bone, resection, neoplasm—all sites and types | 6 |
Brain neoplasm, resection—cerebello‑pontine angle | 4 |
Brain or meninges, biopsy—all lesions | 5 |
Brain or meninges, not neoplasm—temporal lobe | 6 |
Brain or meninges, resection—neoplasm (intracranial) | 5 |
Brain or meninges, resection—not neoplasm | 4 |
Branchial cleft, cyst | 4 |
Breast, excision biopsy, guidewire localisation—non‑palpable lesion | 6 |
Breast, excision biopsy, or radical resection, malignant neoplasm or atypical proliferative disease—all specimen types | 6 |
Breast, incision biopsy or needle biopsy, malignant neoplasm—all specimen types | 4 |
Breast, microdochectomy | 6 |
Breast, orientated wide local excision for carcinoma with margin assessment | 7 |
Breast tissue—all specimens not otherwise specified | 4 |
Bronchus, biopsy | 4 |
Carotid body—neoplasm | 5 |
Cholesteatoma | 3 |
Digits, amputation—not traumatic | 4 |
Digits, amputation—traumatic | 2 |
Ear, middle and inner—not cholesteatoma | 4 |
Endocrine neoplasm—not otherwise specified | 5 |
Extremity, amputation—not otherwise specified | 4 |
Extremity, amputation or disarticulation—neoplasm | 6 |
Eye, conjunctiva—biopsy or pterygium | 3 |
Eye, cornea | 4 |
Eye, enucleation or exenteration—all lesions | 6 |
Eye—not otherwise specified | 4 |
Fallopian tube, biopsy | 4 |
Fallopian tube, ectopic pregnancy | 4 |
Fallopian tube, sterilisation | 2 |
Fetus with dissection | 6 |
Foreskin—new born | 2 |
Foreskin—not new born | 3 |
Gallbladder | 3 |
Gallbladder and porta hepatis‑radical resection | 6 |
Ganglion cyst, all sites | 3 |
Gum or oral mucosa, biopsy | 4 |
Heart—not otherwise specified | 5 |
Heart valve | 4 |
Hernia sac | 2 |
Hydrocele sac | 2 |
Jaw, upper or lower, including bone—radical resection for neoplasm | 6 |
Joint and periarticular tissue, without bone—all specimens | 3 |
Joint tissue, including bone—all specimens | 4 |
Kidney, biopsy including transplant | 5 |
Kidney, nephrectomy transplant | 5 |
Kidney, partial or total nephrectomy—not neoplasm | 4 |
Kidney, partial or total nephrectomy or nephroureterectomy—neoplasm | 6 |
Large bowel, colostomy—stoma | 3 |
Large bowel (including rectum), biopsy—all sites | 4 |
Large bowel (including rectum), biopsy, for confirmation or exclusion of Hirschsprung’s Disease | 5 |
Large bowel (including rectum), polyp | 4 |
Large bowel (including rectum), segmental resection—neoplasm | 6 |
Large bowel (including rectum), submucosal resection—neoplasm | 5 |
Large bowel, segmental resection—colon, not neoplasm | 5 |
Larynx, biopsy | 4 |
Larynx, partial or total resection | 5 |
Larynx, resection with nodes or pharynx or both | 6 |
Lip biopsy—all specimens not mentioned | 3 |
Lip wedge resection or local excision with orientation | 4 |
Liver—all specimens not otherwise specified | 5 |
Liver, hydatid cyst or resection for trauma | 4 |
Liver, total or subtotal hepatectomy—neoplasm | 6 |
Lung, needle or transbronchial biopsy | 4 |
Lung, resection—neoplasm | 6 |
Lung segment, lobar or total resection | 6 |
Lung, wedge biopsy | 5 |
Lymph node, biopsy—all sites | 4 |
Lymph node, biopsy, for lymphoma or lymphoproliferative disorder | 5 |
Lymph nodes, regional resection—all sites | 5 |
Mediastinum mass | 5 |
Muscle, biopsy | 6 |
Nasopharynx or oropharynx, biopsy | 4 |
Nerve, biopsy neuropathy | 5 |
Nerve, neurectomy or removal of neoplasm | 4 |
Nerve—not otherwise specified | 3 |
Nose, mucosal biopsy | 4 |
Nose or sinuses, polyps | 3 |
Odontogenic neoplasm | 5 |
Odontogenic or dental cyst | 4 |
Oesophagus, biopsy | 4 |
Oesophagus, diverticulum | 3 |
Oesophagus, partial or total resection | 6 |
Oesophagus, submucosal resection—neoplasm | 5 |
Omentum, biopsy | 4 |
Ovary with or without tube—neoplasm | 5 |
Ovary with or without tube—not neoplasm | 4 |
Pancreas, biopsy | 5 |
Pancreas, cyst | 4 |
Pancreas, subtotal or total with or without splenectomy | 6 |
Parathyroid gland(s) | 4 |
Penisectomy—simple | 4 |
Penisectomy with node dissection | 5 |
Peritoneum, biopsy | 4 |
Pituitary neoplasm | 4 |
Placenta—not third trimester | 4 |
Placenta—third trimester, abnormal pregnancy or delivery | 4 |
Pleura or pericardium, biopsy or tissue | 4 |
Products of conception, spontaneous or missed abortion | 4 |
Products of conception, termination of pregnancy | 3 |
Prostate—all types of specimen not otherwise specified | 4 |
Prostate, radical prostatectomy or cystoprostatectomy for carcinoma | 7 |
Prostate, radical resection | 6 |
Retroperitoneum, neoplasm | 5 |
Salivary gland—all specimens not otherwise specified | 4 |
Salivary gland, Mucocele | 3 |
Salivary gland, neoplasm—all sites | 5 |
Sinus, paranasal, biopsy | 4 |
Sinus, paranasal, resection—neoplasm | 6 |
Skin—all specimens not otherwise specified including all neoplasms and cysts | 3 |
Skin, biopsy—blistering skin diseases | 4 |
Skin, biopsy—inflammatory dermatosis | 4 |
Skin, biopsy—investigation of alopecia where serial horizontal sections are taken, except for male pattern baldness | 5 |
Skin, biopsy—investigation of lymphoproliferative disorder | 5 |
Skin, eyelid, wedge resection | 4 |
Skin, local resection—orientation | 4 |
Skin, resection of malignant melanoma or melanoma in situ | 5 |
Small bowel—all specimens not otherwise specified | 5 |
Small bowel—biopsy, all sites | 4 |
Small bowel, diverticulum | 3 |
Small bowel, resection—neoplasm | 6 |
Small bowel, submucosal resection—neoplasm | 5 |
Soft tissue, infiltrative lesion—extensive resections at least 5 cm in maximal dimension | 6 |
Soft tissue, lipoma and variants | 3 |
Soft tissue, neoplasm, not lipoma—all specimens | 5 |
Soft tissue—not otherwise specified | 4 |
Spleen | 5 |
Stomach—all specimens not otherwise specified | 4 |
Stomach, endoscopic biopsy or endoscopic polypectomy | 4 |
Stomach, resection, neoplasm—all specimens | 6 |
Stomach, submucosal resection—neoplasm | 5 |
Tendon or tendon sheath, giant cell neoplasm | 4 |
Tendon or tendon sheath—not otherwise specified | 3 |
Testis and adjacent structures, castration | 2 |
Testis and adjacent structures, neoplasm with or without nodes | 5 |
Testis and adjacent structures—not otherwise specified | 3 |
Testis and adjacent structures, vas deferens sterilisation | 2 |
Testis, biopsy | 5 |
Thymus—not otherwise specified | 5 |
Thyroglossal duct—all lesions | 4 |
Thyroid—all specimens | 5 |
Tissue or organ—all specimens not otherwise specified | 3 |
Tissue or organ not otherwise specified, abscess | 3 |
Tissue or organ not otherwise specified, haematoma | 3 |
Tissue or organ not otherwise specified, malignant neoplasm with regional nodes | 6 |
Tissue or organ not otherwise specified, neoplasm local | 4 |
Tissue or organ not otherwise specified, pilonidal cyst or sinus | 3 |
Tissue or organ not otherwise specified, thrombus or embolus | 3 |
Tissue or organ not otherwise specified, veins varicosity | 3 |
Tongue, biopsy | 4 |
Tongue or tonsil, neoplasm local | 5 |
Tongue or tonsil, neoplasm with nodes | 6 |
Tonsil, biopsy—excluding resection of whole organ | 4 |
Tonsil or adenoids or both | 2 |
Trachea, biopsy | 4 |
Ureter, biopsy | 4 |
Ureter, resection | 5 |
Urethra, biopsy | 4 |
Urethra, resection | 5 |
Urinary bladder—all specimens not otherwise specified | 4 |
Urinary bladder, partial or total with or without prostatectomy | 6 |
Urinary bladder, transurethral resection of neoplasm | 5 |
Uterus and/or cervix—all specimens not otherwise specified | 4 |
Uterus, cervix cone, biopsy (including LEEP or LLETZ biopsy) | 5 |
Uterus, cervix, curettings or biopsy | 4 |
Uterus, endocervix, polyp | 3 |
Uterus, endometrium, polyp | 3 |
Uterus, with or without adnexa, malignant neoplasm—all specimen types not otherwise specified | 6 |
Uterus with or without adnexa, neoplasm, Wertheim’s or pelvic clearance | 6 |
Vagina, biopsy | 4 |
Vaginal mucosa, incidental | 3 |
Vagina, radical resection | 6 |
Vulva or labia, biopsy | 4 |
Vulval, subtotal or total with or without nodes | 6 |
Part 5—Dictionary
Note: All references in the Dictionary to a provision are references to a provision in this Schedule, unless otherwise indicated.
In this Schedule:
abnormal level of TSH, for item 66719, has the meaning given by clause 2.2.5.
Act means the Health Insurance Act 1973.
approved collection centre, for Group P10, has the meaning given by clause 2.10.1.
ASGC, for item 74991, has the meaning given by subclause 2.12.1(3).
biopsy material, for Group P5, has the meaning given by clause 2.5.1.
bulk‑billed, for items 74990 and 74991, has the meaning given by subclause 2.12.1(2).
Commonwealth concession card holder, for items 74990 and 74991, has the meaning given by subclause 2.12.1(2).
compatibility tests by crossmatch, for items 65099 and 65102, has the meaning given by clause 2.1.2.
cytology, for Group P5, has the meaning given by clause 2.5.1.
designated test, has the meaning given by clause 1.2.4.
elevated serum ferritin, for items 73317 and 73318, has the meaning given by clause 2.7.1.
general practitioner, has the meaning given by clause 1.2.6.
group, for a group in this table, means every item in the group.
Hepatitis C sero‑positive, for items 69499 and 69500, has the meaning given by clause 2.3.5.
institution, for Group P10, has the meaning given by clause 2.10.1.
item means:
(a) an item mentioned, by number, in column 1 of a table in:
(i) this Schedule; or
(ii) the diagnostic imaging services table; or
(iii) the general medical services table; or
(b) in a reference immediately followed by a number—the item so numbered.
metal toxicity testing group, has the meaning given by clause 2.2.7.
nutritional metals testing group, has the meaning given by clause 2.2.7.
patient episode means:
(a) a pathology service or pathology services (other than a pathology service to which paragraph (b) refers) provided for a single patient whose need for the service or services was determined under section 16A of the Act:
(i) on the same day; or
(ii) if more than one test is performed on the one specimen within 14 days—on the same or different days;
whether the services:
(iii) are requested by one or more practitioners, participating midwives or participating nurse practitioners; or
(iv) are described in a single item or in more than one item; or
(v) are rendered by one approved pathology practitioner or more than one approved pathology practitioner; or
(vi) are rendered on the same or different days; or
(b) a pathology service to which clause 1.2.3, 2.1.1 or 2.2.2 refers that is provided in the circumstances, set out in the clause, that relate to the service.
Pharmaceutical Benefits Scheme means the scheme for the supply of pharmaceutical benefits established under Part VII of the National Health Act 1953.
practice location, for item 74991, has the meaning given by subclause 2.12.1(3).
prescribed laboratory, for Group P10, has the meaning given by clause 2.10.1.
receiving APP, for a patient episode, means an approved pathology practitioner in an approved pathology authority who:
(a) receives a request from a referring APP to render a designated test or tests; and
(b) renders each test included in the designated test that the referring APP has not performed.
recognised pathologist means:
(a) a medical practitioner recognised as a specialist in pathology under subsection 3D(1) of the Act; or
(b) a medical practitioner in relation to whom there is in force a determination under paragraph 3DB(4)(a) or subsection 3E(1) of the Act that the practitioner is recognised as a specialist in pathology.
referring APP, for a patient episode, means an approved pathology practitioner in an approved pathology authority who:
(a) has received a request to render one or more designated tests; and
(b) is unable, because of the lack of facilities in, or expertise or experience of the staff of, the laboratory of the authority, to render one or more of the tests included in the designated test; and
(c) requests a receiving APP in another approved pathology authority to render:
(i) the test or tests that the approved pathology practitioner is unable to render; or
(ii) all of the tests that are included in the designated test; and
(d) renders each test included in the designated test, other than the test or tests for which the request mentioned in paragraph (c) is made.
regional, rural or remote area, for item 74991, has the meaning given by subclause 2.12.1(3).
request, received by an approved pathology practitioner, includes a request for a pathologist‑determinable service to which subsection 16A(6) of the Act applies.
residential care facility, for Group P10, has the meaning given by clause 2.10.1.
rule 3 exemption means the exemption that has effect because of the operation of clause 1.2.2.
separately identified specimen, for Group P5, has the meaning given by clause 2.5.1.
serial examinations means a series of examinations requested on one occasion, whether or not:
(a) the materials are received on different days by the approved pathology practitioner; or
(b) the examinations or cultures were requested on one or more request forms by the treating practitioner.
serial examinations or cultures, for Group P3, has the meaning given by clause 2.3.1.
serological status is uncertain, for items 69499 and 69500, has the meaning given by clause 2.3.5.
set of pathology services, has the meaning given by clause 1.2.7.
SLA, for item 74991, has the meaning given by subclause 2.12.1(3).
specimen collection centre, for Group P10, has the meaning given by clause 2.10.1.
SSD for item 74991, has the meaning given by subclause 2.12.1(3).
treating practitioner, for Group P10, has the meaning given by clause 2.10.1.
unreferred service, for items 74990 and 74991, has the meaning given by subclause 2.12.1(3).
