![]()
Food Standards (Proposal P1040 – Code Revision – Consequential & Corrective Amendments II) Variation
The Board of Food Standards Australia New Zealand gives notice of the making of this variation under section 92 of the Food Standards Australia New Zealand Act 1991. The variation commences on the dates specified in clause 3 of this variation.
Dated 16 February 2016

Standards Management Officer
Delegate of the Board of Food Standards Australia New Zealand
Note:
This variation will be published in the Commonwealth of Australia Gazette No. FSC 103 on 22 February 2016. This means that this date is the gazettal date for the purposes of clause 3 of the variation.
1 Name
This instrument is the Food Standards (Proposal P1040 – Code Revision – Consequential & Corrective Amendments II) Variation.
2 Variation to standards in the Australia New Zealand Food Standards Code
The Schedule varies standards in the Australia New Zealand Food Standards Code.
3 Commencement
(1) Subject to subsection (2), the variation commences on 1 March 2016 immediately after the commencement of Standard 5.1.1 – Revocation and transitional provisions – 2014 Revision.
(2) Items 1 and 4 of the Schedule commence on 19 January 2017.
Schedule
Standard 1.1.1 – Structure of the Code and general provisions
[1] Subsection 1.1.1—2(2)
Omit ‘Standard 1.2.12 – Transitional standard for dietary fibre nutrition content claims’
Standard 1.1.2 – Definitions used throughout the Code
[2] Subsection 1.1.2—2(3) (definition of individual portion pack)
Omit ‘1.2.1—6(4)’, substitute ‘1.2.1—6(3)’
[3] Section 1.1.2—12 (Note)
Omit ‘S28—2, 0, S29—18’, substitute ‘S28—2, S29—18’
Standard 1.2.7 – Nutrition, health and related claims
[4] Section 1.2.7—12 (Note)
Standard 1.3.1 – Food additives
[4A] Section 1.3.1—2 (Note)
Omit ‘that that’, substitute ‘that’
Standard 1.4.1 – Contaminants and natural toxicants
[5] Subsection 1.4.1—3(3)
Omit
![]()
substitute
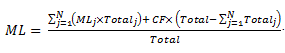
Standard 1.4.2 – Agvet chemicals
[6] Standard Heading (Note 3)
Omit ‘2014’, substitute ‘2014.’
Standard 1.5.2 – Food produced using gene technology
[7] Standard Heading (Note 3)
Omit ‘1.1.1—10(3)(c) and (4)(g)’, substitute ‘1.1.1—10(5)(c) and (6)(g)’
Standard 2.4.2 – Edible oil spreads
[8] Section 2.4.2—2 (Note)
Omit ‘edible oil spread’, substitute ‘edible oil spread’
Standard 2.7.1 – Labelling of alcoholic beverages and food containing alcohol
[9] Section 2.7.1—1
Omit ‘Alcoholic beverages’, substitute ‘Labelling of alcoholic beverages and food containing alcohol’
Standard 2.7.4 – Wine and wine product
[10] Standard Heading (Note 3)
Omit ‘the Wine Australia Corporation Act 1980 (Cth)’, substitute ‘the Australian Grape and Wine Authority Act 2013 (Cth)’
Standard 2.9.4 – Formulated supplementary sports foods
[11] Paragraph 2.9.4—6(2)(a)
Omit ‘of reconstitution’, substitute ‘or reconstitution’
Standard 2.9.5 – Food for special medical purposes
[12] Paragraph 2.9.5—3(b)
Omit ‘Part 2’, substitute ‘Part 1.2’
Standard 2.9.6 – Transitional standard for special purpose foods (including amino acid modified foods)
[13] Section 2.9.6—3 (Note)
Omit ‘published’, substitute ‘published.’
Standard 2.10.2 – Salt and salt products
[14] Section 2.10.2—3
Omit all text after the words ‘A food’, substitute ‘that is sold as ‘salt’ must be salt and contain no less than 970 g/kg sodium chloride on a dry basis, exclusive of permitted additives.’
Schedule 1 – RDIs and ESADDIs
[15] Section S1—2 (table)
Omit
Vitamin E | RDI | 10 mg alpha- tocopherol equivalents4 | 5 mg alpha- tocopherol equivalents4 | 4 mg alpha- tocopherol equivalents4 |
substitute
Vitamin E | RDI | 10 mg alpha- tocopherol equivalents3 | 5 mg alpha- tocopherol equivalents3 | 4 mg alpha- tocopherol equivalents3 |
[15A] Section S1—2 (Notes)
Omit
Note 1 See paragraph 1.1.2—14(a).
Note 2 See paragraph 1.1.2—14(b).
Note 3 See paragraph 1.1.2—14(c).
Note 4 See paragraph 1.1.2—14(d).
substitute
Note 1 See paragraph 1.1.2—14(3)(a).
Note 2 See paragraph 1.1.2—14(3)(b).
Note 3 See paragraph 1.1.2—14(3)(c).
Schedule 2 – Units of measurement
[16] Section S2—2 (table)
Omit ‘mJ’, substitute ‘MJ’
Schedule 3 – Identity and Purity
[16A] Section S3—27
Omit ‘cfu/kg’, substitute ‘cfu/g’
Schedule 4 – Nutrition, health and related claims
[17] Section S4—2 (Note - definition of sugars)
Omit ‘(a)’ (second occurring), substitute ‘(b)’
[18] Section S4—5 (table)
(a) Omit
Iodine | Contributes to normal growth and development | Children |
|
|
substitute
| Contributes to normal growth and development | Children |
|
|
Selenium | Contributes to the maintenance of normal hair and nails |
|
|
|
substitute
| Contributes to the maintenance of normal hair and nails |
|
|
|
(c) Omit
Energy | Contributes to weight loss or weight maintenance |
| Diet reduced in energy and including regular exercise | The food: (a) meets the conditions for making a ‘diet’ nutrition content claim; or (b) is a formulated meal replacement and contains no more than 1200 kJ per serving |
substitute
| Contributes to weight loss or weight maintenance |
| Diet reduced in energy and including regular exercise | The food: (a) meets the conditions for making a ‘diet’ nutrition content claim; or (b) is a formulated meal replacement and contains no more than 1200 kJ per serving |
Schedule 12 – Nutrition information panels
[19] Section S12—4 (table)
Omit ‘Your daily intakes may be higher or lower depending on your energy needs.’
Schedule 15 – Substances that may be used as food additives
[20] Section S15—5 (table)
(a) Omit the following from item 1.4.2 (where second occurring)
234 | Nisin | 10 |
|
475 | Polyglycerol esters of fatty acids | 5 000 | Only whipped thickened light cream |
(b) Insert in item 2.2.2 in numerical order
200 201 202 203 | Sorbic acid and sodium, potassium and calcium sorbates | 2 000 |
|
Schedule 18 – Processing aids
[21] Section S18—3 (table)
Omit
Diethylenetriamine, triethylene-tetramine, or tetraethylenepentamin cross-linked with epichlorohydrin | GMP |
substitute
Diethylenetriamine, triethylene-tetramine, or tetraethylenepentamine cross-linked with epichlorohydrin | GMP |
Schedule 26 – Food produced using gene technology
[22] Schedule Heading (Note 1)
Omit ‘1.1.1—10(3)(c) and (4)(g)’, substitute ‘1.1.1—10(5)(c) and (6)(g)’
[23] Subsection S26—3(4) (table)
(a) Omit
4 | Lucerne | (a) herbicide-tolerant lucerne lines J101 & J163 |
substitute
4 | Lucerne | (a) herbicide-tolerant lucerne lines J101 and J163 |
(b) Omit
|
| (b) food derived from reduced lignin lucerne line KK179 |
substitute
|
| (b) reduced lignin lucerne line KK179 |
Schedule 29 – Special purpose foods
[24] Section S29—17 (Table heading)
Omit ‘and intake amounts’
[24A] Section S29—21 (Notes)
Omit
Note 1 See paragraph 1.1.2—14(3)(a)
Note 2 For niacin, add niacin and any niacin provided from the conversion of the amino acid tryptophan, using the conversion factor 1:60.
Note 3 See paragraph 1.1.2—14(3)(d)
substitute
Note 1 See paragraph 1.1.2—14(3)(a).
Note 2 For niacin, add niacin and any niacin provided from the conversion of the amino acid tryptophan, using the conversion factor 1:60.
Note 3 See paragraph 1.1.2—14(3)(c).