1 Name
(1) This instrument is the National Health (Commonwealth Price—Pharmaceutical Benefits Supplied By Public Hospitals) Amendment (Budget Measure) Determination 2019.
(2) This instrument may also be cited as PB 57 of 2019.
2 Commencement
(1) Each provision of this instrument specified in column 1 of the table commences, or is taken to have commenced, in accordance with column 2 of the table. Any other statement in column 2 has effect according to its terms.
Commencement information |
Column 1 | Column 2 | Column 3 |
Provisions | Commencement | Date/Details |
1. The whole of this instrument | 1 October 2019. | 1 October 2019 |
Note: This table relates only to the provisions of this instrument as originally made. It will not be amended to deal with any later amendments of this instrument.
(2) Any information in column 3 of the table is not part of this instrument. Information may be inserted in this column, or information in it may be edited, in any published version of this instrument.
3 Authority
This instrument is made under subsection 99(4) of the National Health Act 1953.
4 Schedules
Each instrument that is specified in a Schedule to this instrument is amended or repealed as set out in the applicable items in the Schedule concerned, and any other item in a Schedule to this instrument has effect according to its terms.
Schedule 1—Amendments
National Health (Commonwealth Price—Pharmaceutical Benefits Supplied By Public Hospitals) Determination 2017
1 After section 2D
Insert:
2E Application and transitional arrangements
Schedule 1 sets out application and transitional arrangements in relation to amendments of this instrument.
2 Section 3
Insert:
determined quantity, of a listed brand of a pharmaceutical item, has the same meaning as in Part VII of the Act.
maximum quantity, of a brand of a pharmaceutical item, means a quantity or number of units of the pharmaceutical item determined under paragraph 85A(2)(a) of the Act in relation to that brand of pharmaceutical item.
3 Paragraph 9(a)
Repeal the paragraph, substitute:
(a) if the quantity of the benefit supplied is equal to a multiple of a pack quantity of the benefit—the sum of:
(i) the approved ex‑manufacturer price or the proportional ex‑manufacturer price (as applicable) for each pack quantity; and
(ii) the mark‑up worked out under section 14 for each pack quantity; or
4 Paragraph 9(c)
Repeal the paragraph, substitute:
(c) if the quantity of the benefit supplied is more than a multiple of a pack quantity of the benefit—the sum of:
(i) the approved ex‑manufacturer price or the proportional ex‑manufacturer price (as applicable) for each pack quantity; and
(ii) the mark‑up worked out under section 14 for each pack quantity; and
(iii) the amount worked out under section 11 in respect of the remainder of the quantity supplied that is a broken quantity.
5 Section 11
Repeal the section, substitute:
11 Dispensed price—broken quantities
If a broken quantity is supplied, the amount referred to in paragraph 9(b) or subparagraph 9(c)(iii) is worked out using the following method statement.
Method statement
Step 1. Work out the mark‑up on the approved ex‑manufacturer price or proportional ex‑manufacturer price (as applicable) for the pack quantity in accordance with section 14.
Step 2. Add that mark‑up to the approved ex‑manufacturer price or proportional ex‑manufacturer price (as applicable) for the pack quantity.
Step 3. Ascertain the percentage that the broken quantity bears to the pack quantity.
Step 4. Take that percentage of the amount worked out under step 2 (the resulting amount is the dispensed price for the supply of the broken quantity).
6 At the end of Part 3
Add:
14 Dispensed price—mark‑up on approved ex‑manufacturer price or proportional ex‑manufacturer price
(1) For the purposes of subparagraphs 9(a)(ii) and (c)(ii) and step 1 in section 11, the mark‑up for a pack quantity of a ready‑prepared pharmaceutical benefit is worked out in accordance with this section.
(2) Work out the relevant quantity for the pack quantity, and the ex‑manufacturer price for the relevant quantity, using the following method statement.
Method statement
Step 1. Identify the approved ex‑manufacturer price (the AEMP) or the proportional ex‑manufacturer price (the PEMP) (as applicable) for the pack quantity.
Step 2. Identify any maximum quantities and any determined quantities of each listed brand of the pharmaceutical item concerned (other than any maximum quantity that relates to a supply of any of those brands that can only be made in accordance with special arrangements under section 100 of the Act).
Step 3. From the quantities identified in step 2, identify the relevant quantity for the pack quantity, which is:
(a) the maximum quantity (if any) that is the highest whole number multiple of the pack quantity, or, if there is no such maximum quantity, the determined quantity (if any) that is the highest whole number multiple of the pack quantity; or
(b) if paragraph (a) does not apply—the maximum quantity (if any) that is the closest to the pack quantity or, if 2 maximum quantities are equally close, the greater of those maximum quantities; or
(c) if paragraph (a) does not apply and there are no maximum quantities—the determined quantity (if any) that is the closest to the pack quantity or, if 2 determined quantities are equally close, the greater of those determined quantities.
Step 4. The ex‑manufacturer price for the relevant quantity is the relevant quantity multiplied by the AEMP or PEMP (as applicable) for the pack quantity.
(3) If the ex‑manufacturer price for the relevant quantity is $930.06 or less, the mark‑up for the pack quantity is 7.52% of the approved ex‑manufacturer price or the proportional ex‑manufacturer price (as applicable) for the pack quantity.
(4) If:
(a) the ex‑manufacturer price for the relevant quantity is more than $930.06; and
(b) the relevant quantity and the pack quantity are the same;
the mark‑up for the pack quantity of the brand of the pharmaceutical item is $69.94.
(5) If:
(a) the ex‑manufacturer price for the relevant quantity is more than $930.06; and
(b) the relevant quantity and the pack quantity are not the same;
the mark‑up for the pack quantity is worked out using the following formula:
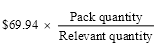
7 At the end of the instrument
Add:
Schedule 1—Application and transitional arrangements
Note: See section 2E.
Part 1—Amendments made by the National Health (Commonwealth Price—Pharmaceutical Benefits Supplied By Public Hospitals) Amendment (Budget Measure) Determination 2019
1 Application of amendments
The amendments of Part 3 of this instrument by the National Health (Commonwealth Price—Pharmaceutical Benefits Supplied By Public Hospitals) Amendment (Budget Measure) Determination 2019 apply in relation to the supply of a pharmaceutical benefit on or after 1 October 2019.