Part 1—Preliminary
1 Name
(1) This instrument is the National Health (Commonwealth Price - Pharmaceutical benefits supplied by private hospitals) Determination 2020.
(2) This instrument may also be cited as PB 99 of 2020.
2 Commencement
(1) Each provision of this instrument specified in column 1 of the table commences, or is taken to have commenced, in accordance with column 2 of the table. Any other statement in column 2 has effect according to its terms.
Commencement information |
Column 1 | Column 2 | Column 3 |
Provisions | Commencement | Date/Details |
1. The whole of this instrument | 1 October 2020 | 1 October 2020 |
Note: This table relates only to the provisions of this instrument as originally made. It will not be amended to deal with any later amendments of this instrument.
(2) Any information in column 3 of the table is not part of this instrument. Information may be inserted in this column, or information in it may be edited, in any published version of this instrument.
2A Authority
This instrument is made under subsection 99(4) of the National Health Act 1953.
2B Purpose
The purpose of this determination is to determine the amount of the Commonwealth payment for pharmaceutical benefits supplied by an approved hospital authority to a patient receiving treatment in or at a private hospital for which the authority is approved.
2C Schedules
Each instrument that is specified in a Schedule to this instrument is amended or repealed as set out in the applicable items in the Schedule concerned, and any other item in a Schedule to this instrument has effect according to its terms.
2D Things done under the National Health (Pharmaceutical benefits supplied by private hospitals) Determination 2010 (21/09/2010)
(1) If:
(a) a thing was done for a particular purpose under the National Health Act 1953 ‑ National Health (Pharmaceutical benefits supplied by private hospitals) Determination 2010 (21/09/2010) as in force immediately before that Determination was repealed; and
(b) the thing could be done for that purpose under this instrument;
the thing has effect for the purposes of this instrument as if it had been done under this instrument.
(2) Without limiting subsection (1), a reference in that subsection to a thing being done includes a reference to a notice, application or other instrument being given or made.
3 Definitions
In this instrument:
Act means the National Health Act 1953.
approved ex‑manufacturer price has the same meaning as in subsection 84(1) of the Act.
approved hospital authority means a hospital authority that has been approved by the Minister under subsection 94(1) of the Act.
Approved Pharmacists Determination means the determination made under paragraph 98B(1)(a) of the Act, as it applies at the time that a pharmaceutical benefit is supplied.
brand has the same meaning as in Part VII of the Act.
current figure has the same meaning as in section 99G of the Act.
dangerous drug means:
(a) a pharmaceutical benefit mentioned in Schedule 3 to the National Health (Commonwealth Price and Conditions for Commonwealth Payments for Supply of Pharmaceutical Benefits) Determination 2019 (PB 114 of 2019); or
(b) a pharmaceutical benefit that, under the law of a State or Territory, is classified as a dangerous drug.
dangerous drug fee has the same meaning as in Approved Pharmacists Determination.
determined quantity, of a listed brand of a pharmaceutical item, has the same meaning as in Part VII of the Act.
dispensed price, for a pharmaceutical benefit, has the meaning given by section 11.
extemporaneously‑prepared dispensing fee has the same meaning as in the Approved Pharmacists Determination.
extemporaneously‑prepared pharmaceutical benefit means a pharmaceutical benefit that is not a ready‑prepared pharmaceutical benefit.
listed brand has the same meaning as in Part VII of the Act.
maximum quantity, of a brand of a pharmaceutical item, means a quantity or number of units of the pharmaceutical item determined under paragraph 85A(2)(a) of the Act in relation to that brand of pharmaceutical item.
pack quantity has the same meaning as in subsection 84(1) of the Act.
Note: The Minister may determine one or more pack quantities for a brand of a pharmaceutical item under subsection 84AK(2) of the Act. The quantities determined are the quantities in manufacturer’s PBS packs.
patient co‑payment means:
(a) for a person to whom, at the time that a pharmaceutical benefit is supplied, paragraph 87(2)(b) of the Act applies—the current figure for the general patient reduced charge, as defined in section 99F of the Act; or
(b) for a person who is a concessional beneficiary for section 84 of the Act—the current figure for the concessional beneficiary charge, as defined in section 99F of the Act; or
(c) for any other person—the current figure for the general patient charge, as defined in section 99F of the Act.
pharmaceutical benefit has the meaning given by section 84 of the Act.
pharmaceutical item has the same meaning as in Part VII of the Act.
private hospital means a hospital for which there is in force a statement under subsection 121‑5(8) of the Private Health Insurance Act 2007 that the hospital is a private hospital.
proportional ex‑manufacturer price has the same meaning as in subsection 84(1) of the Act.
ready‑prepared dispensing fee has the same meaning as in the Approved Pharmacists Determination.
ready‑prepared pharmaceutical benefit means a brand of a pharmaceutical item for which there is a determination under subsection 85(6) of the Act.
Regulations means the National Health (Pharmaceutical Benefits) Regulations 2017.
repatriation pharmaceutical benefit has the meaning given by section 84 of the Act.
Part 2—Rates and conditions of payment—general
4 Rounding up and rounding down
If the calculation of a price under this Determination includes a fraction of a cent, the final amount calculated is to then be rounded up or down to the nearest cent, with an amount of 0.5 of a cent or more being rounded up to the next cent.
5 Dangerous drug fee
If a pharmaceutical benefit is a dangerous drug, the Commonwealth must pay, in addition to any other amounts under this Determination, a dangerous drug fee where indicated in this Determination.
6 Repeat supply
(1) If, under subsection 88(6) of the Act and section 49 of the Regulations, a medical practitioner, instead of directing a repeated supply of a pharmaceutical benefit, directs the supply of a quantity or number of units of the benefit on 1 occasion, not exceeding the total quantity or number of units that could be prescribed if the medical practitioner directed a repeated supply, the price for the purpose of this Determination for the supply includes:
(a) only 1 dispensing fee, that may be:
(i) a ready‑prepared dispensing fee; or
(ii) an extemporaneously‑prepared dispensing fee; and
(b) the price, if any, of only 1 container; and
(c) if a dangerous drug fee applies, only one such fee.
(2) For this section, the price, if any, for a container is to be worked out under section 12.
7 Drugs and medicinal preparations to which a subsection 85(6) determination applies
If a determination under subsection 85(6) of the Act applies to a pharmaceutical benefit that is a drug or medicinal preparation, the Commonwealth will only make a payment for the supply of a drug or medicinal preparation of the brand mentioned in the determination.
Part 3—Dispensed price for supply of ready‑prepared pharmaceutical benefits
8 Amount payable to approved hospital authority
(1) The amount payable to an approved hospital authority for the supply of a ready‑prepared pharmaceutical benefit to a patient receiving treatment in or at a private hospital for which the hospital authority is approved is the amount by which the dispensed price for the benefit exceeds the patient co‑payment.
(2) For a person to whom, at the time that the benefit is supplied, subsection 87(5A) of the Act applies, the amount of the patient co‑payment is zero.
9 Dispensed price—general
(1) The dispensed price for the supply of a ready‑prepared pharmaceutical benefit by an approved hospital authority to a patient receiving treatment in or at a private hospital for which the authority is approved is:
(a) if a quantity of the benefit that is ordered and supplied is equal to a multiple of a pack quantity of the benefit—the sum of:
(i) for each pack quantity:
(A) the approved ex‑manufacturer price or the proportional ex‑manufacturer price (as applicable) for the pack quantity; and
(B) the storage and handling mark‑up worked out under section 11A for the pack quantity; and
(C) the mark‑up, worked out under section 12; and
(ii) a ready‑prepared dispensing fee; and
(iii) if a dangerous drug fee applies, the dangerous drug fee; or
(b) if a quantity of the benefit that is ordered and supplied is less than a pack quantity of the benefit—the sum of:
(i) the amount worked out under section 14; and
(ii) a ready‑prepared dispensing fee; and
(iii) if a dangerous drug fee applies, the dangerous drug fee; and
(iv) an amount for the supply of a container, worked out under section 13; or
(c) if a quantity of the benefit that is ordered and supplied is more than a multiple of a pack quantity of the benefit—the sum of:
(i) for each pack quantity:
(A) the approved ex‑manufacturer price or the proportional ex‑manufacturer price (as applicable) for the pack quantity; and
(B) the storage and handling mark‑up worked out under section 11A for the pack quantity; and
(C) the mark‑up worked out under section 12; and
(ii) for the remainder of the quantity that is less than a pack quantity—the amount worked out under section 14; and
(iii) a ready‑prepared dispensing fee; and
(iv) if a dangerous drug fee applies, the dangerous drug fee.
(2) However, for a ready‑prepared pharmaceutical benefit that comprises the admixture of ready‑prepared ingredients and is specified in Schedule 1 to the National Health (Commonwealth Price and Conditions for Commonwealth Payments for Supply of Pharmaceutical Benefits) Determination 2019:
(a) the ready‑prepared dispensing fee does not apply; and
(b) an extemporaneously‑prepared dispensing fee must be paid by the Commonwealth; and
(c) no amount for the supply of the container is payable; and
(d) if a dangerous drug fee applies, the dangerous drug fee.
10 Storage and handling mark‑up
(1) For the purposes of sub‑subparagraphs 9(1)(a)(i)(B) and (c)(i)(B) and paragraph (b) of step 1 in section 13, the storage and handling mark‑up for a pack quantity of a ready‑prepared pharmaceutical benefit is worked out in accordance with this section.
(2) Work out the relevant quantity for the pack quantity, and the ex‑manufacturer price for the relevant quantity, using the following method statement.
Method statement
Step 1. Identify the approved ex‑manufacturer price (the AEMP) or the proportional ex‑manufacturer price (the PEMP) (as applicable) for the pack quantity.
Step 2. Identify any maximum quantities and any determined quantities of each listed brand of the pharmaceutical item concerned (other than any maximum quantity that relates to a supply of any of those brands that can only be made in accordance with special arrangements under section 100 of the Act).
Step 3. From the quantities identified in step 2, identify the relevant quantity for the pack quantity, which is:
(a) the maximum quantity (if any) that is the highest whole number multiple of the pack quantity, or, if there is no such maximum quantity, the determined quantity (if any) that is the highest whole number multiple of the pack quantity; or
(b) if paragraph (a) does not apply—the maximum quantity (if any) that is the closest to the pack quantity or, if 2 maximum quantities are equally close, the greater of those maximum quantities; or
(c) if paragraph (a) does not apply and there are no maximum quantities—the determined quantity (if any) that is the closest to the pack quantity or, if 2 determined quantities are equally close, the greater of those determined quantities.
Step 4. The ex‑manufacturer price for the relevant quantity is the relevant quantity multiplied by the AEMP or PEMP (as applicable) for the pack quantity.
(3) If the ex‑manufacturer price for the relevant quantity is $930.06 or less, the storage and handling mark‑up for the pack quantity is 7.52% of the approved ex‑manufacturer price or the proportional ex‑manufacturer price (as applicable) for the pack quantity.
(4) If:
(a) the ex‑manufacturer price for the relevant quantity is more than $930.06; and
(b) the relevant quantity and the pack quantity are the same;
the storage and handling mark‑up for the pack quantity is $69.94.
(5) If:
(a) the ex‑manufacturer price for the relevant quantity of the brand of the pharmaceutical item is more than $930.06; and
(b) the relevant quantity and the pack quantity are not the same;
the storage and handling mark‑up for the pack quantity is worked out using the following formula:
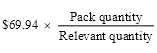
11 Private hospital mark‑up
(1) For sub‑subparagraph 9(1)(a)(i)(C), the mark‑up for a ready‑prepared pharmaceutical benefit is an amount equal to 1.4% of the sum of the amounts worked out under sub‑subparagraphs 9(1)(a)(i)(A) and (B).
(2) For sub‑subparagraph 9(1)(c)(i)(C), the mark‑up for a ready‑prepared pharmaceutical benefit is an amount equal to 1.4% of the sum of the amounts worked out under sub‑subparagraphs 9(1)(c)(i)(A) and (B).
12 Container price
(1) The price for a container for a ready‑prepared pharmaceutical benefit is the sum of:
(a) the wholesale cost worked out under subsection (2); and
(b) the mark‑up worked out under subsection (4).
(2) The wholesale cost for a container will be based on the average of wholesale costs for a particular container, in a quantity of 100, as agreed by the Minister and the Pharmacy Guild of Australia for the supply of the container, by a wholesale drug distributor.
(3) The wholesale cost must be agreed on or before 15 June in a year and takes effect on 1 August in that year.
(4) For paragraph (1)(b), the mark‑up is 10% of the amount agreed under subsection (2).
(5) For a mark‑up worked out under subsection (4), if the calculation of a percentage of the wholesale cost includes a fraction of a cent, the mark‑up is to be rounded up or down to the nearest cent, with an amount of 0.5 of a cent or more being rounded up to the next cent.
(6) In this section:
container means:
(a) for a ready‑prepared pharmaceutical benefit that is injectable—a vial with a capacity of 150 ml; or
(b) for any other ready‑prepared pharmaceutical benefit—a vial with a capacity of 25 ml.
13 Price for broken quantities
If a ready‑prepared pharmaceutical benefit is ordered and supplied in a quantity that is less than a pack quantity (the broken quantity), the amount mentioned in subparagraph 9(1)(b)(i) or (c)(ii) is to be worked out using the following method statement.
Method statement
Step 1 Add together, for a pack quantity:
(a) the approved ex‑manufacturer price or the proportional ex‑manufacturer price (as applicable) for the pack quantity; and
(b) the storage and handling mark‑up worked out under section 10 for the pack quantity; and
(c) the mark‑up worked out under section 11.
Step 2 Divide the quantity or number of units in the broken quantity by the pack quantity and express as a percentage.
Step 3 For a percentage up to and including an amount in column 1 of the following table, select the percentage mentioned in column 2 of the previous item in the following table.
Column 1
Up to and including: (%) | Column 2
Select amount: (%) |
5 | 10 |
10 | 18 |
15 | 26 |
20 | 32 |
25 | 38 |
30 | 44 |
35 | 50 |
40 | 54 |
45 | 58 |
50 | 62 |
55 | 66 |
60 | 70 |
65 | 74 |
70 | 78 |
75 | 82 |
80 | 86 |
85 | 90 |
90 | 94 |
95 | 98 |
100 | 100 |
Step 4 Multiply the amount worked out under step 3 by the amount worked out under step 1.
14 Ready‑prepared pharmaceutical benefits—limit on the dispensed price
If the dispensed price worked out for the supply of a broken quantity of a ready‑prepared pharmaceutical benefit exceeds the dispensed price for a pack quantity, the dispensed price for the pack quantity is the dispensed price for the broken quantity.
15 Pharmaceutical benefits mentioned in a determination under paragraph 98C(1)(b) of the Act
If a prescription directs the supply of a quantity of a pharmaceutical benefit mentioned in a determination under paragraph 98C(1)(b) of the Act as a pharmaceutical benefit the complete pack of which shall be supplied regardless of any lesser quantity ordered, the dispensed price is the price worked out as if a complete pack was supplied.
Part 4—Dispensed price for supply of extemporaneously‑prepared pharmaceutical benefits
16 Extemporaneously‑prepared pharmaceutical benefits
(1) For an extemporaneously‑prepared pharmaceutical benefit supplied to a patient receiving treatment in or at a private hospital for which the hospital authority is approved, the dispensed price is the Commonwealth price determined for the benefit under the Approved Pharmacists Determination, as if the benefit was supplied by an approved pharmacist.
(2) The amount payable to the approved hospital authority for the supply of an extemporaneously‑prepared pharmaceutical benefit to the patient is the amount by which the dispensed price for the benefit exceeds the patient co‑payment.
(3) For a person to whom, at the time that the benefit is supplied, subsection 87(5A) of the Act applies, the amount of the patient co‑payment is zero.