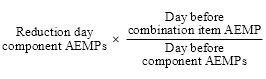1 Name
This instrument is the National Health (Pharmaceutical Benefits) Amendment (2021 Measures No. 1) Regulations 2021.
2 Commencement
(1) Each provision of this instrument specified in column 1 of the table commences, or is taken to have commenced, in accordance with column 2 of the table. Any other statement in column 2 has effect according to its terms.
Commencement information |
Column 1 | Column 2 | Column 3 |
Provisions | Commencement | Date/Details |
1. Sections 1 to 4 and anything in this instrument not elsewhere covered by this table | The day after this instrument is registered. | 17 December 2021 |
2. Schedule 1, Part 1 | 1 July 2022. | 1 July 2022 |
3. Schedule 1, Part 2 | 1 October 2022. | 1 October 2022 |
4. Schedule 1, Part 3 | 1 April 2023. | 1 April 2023 |
5. Schedule 1, Part 4 | 1 July 2023. | 1 July 2023 |
Note: This table relates only to the provisions of this instrument as originally made. It will not be amended to deal with any later amendments of this instrument.
(2) Any information in column 3 of the table is not part of this instrument. Information may be inserted in this column, or information in it may be edited, in any published version of this instrument.
3 Authority
This instrument is made under the National Health Act 1953.
4 Schedules
Each instrument that is specified in a Schedule to this instrument is amended or repealed as set out in the applicable items in the Schedule concerned, and any other item in a Schedule to this instrument has effect according to its terms.
Schedule 1—Amendments
Part 1—Amendments commencing 1 July 2022
National Health (Pharmaceutical Benefits) Regulations 2017
1 Before section 66
Insert:
65A Price reductions for single brands of combination items
(1) This section sets out, for the purposes of subsection 99ACC(2) of the Act, the method for calculating the reduced approved ex‑manufacturer price of a single brand of a combination item on the reduction day mentioned in that subsection.
(2) The reduced approved ex‑manufacturer price of the brand of the combination item is the amount worked out by the following formula:
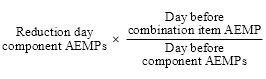
 where:
where:
component drug, in relation to a drug in a combination item, means a drug or medicinal preparation that is contained in that drug.
day before combination item AEMP means the approved ex‑manufacturer price of the brand of the combination item on the day before the reduction day.
day before component AEMPs means the sum of:
(a) the approved ex‑manufacturer prices, on the day before the reduction day, of any one brand of each of the listed component items, adjusted in accordance with subsection (3); and
(b) if the combination item includes one or more component drugs that are not listed component drugs—the non‑listed component price.
differential reduction percentage means:
(a) if there is only one listed component item for which the approved ex‑manufacturer price of any one brand of the listed component item has been reduced under a provision mentioned in subsection 99ACC(6) of the Act on the reduction day—the difference between 100% and the percentage by which the approved ex‑manufacturer price of any one brand of the listed component item in the combination item has been so reduced; or
(b) if there are 2 or more listed component items for which the approved ex‑manufacturer price of any one brand of each of those listed component items has been reduced under a provision mentioned in subsection 99ACC(6) of the Act on the reduction day—the difference between 100% and the average of the percentages by which the approved ex‑manufacturer price of any one brand of each of those listed component items has been so reduced.
listed component drug means a component drug in relation to which a declaration under subsection 85(2) is in force.
listed component item, for each listed component drug contained in the combination item, means the pharmaceutical item that has:
(a) the listed component drug; and
(b) the same manner of administration as the combination item as referred to in subsection 99ACC(7) of the Act; and
(c) subject to subsection (4) of this section, the smallest difference in the total quantity or amount of the listed component drug contained in the quantity or number of units in the pricing quantity of any one brand of the pharmaceutical item compared to the total quantity or amount of the listed component drug in the pricing quantity of the brand of the combination item.
non‑listed component price means the day before combination item AEMP reduced (but not below zero) by the sum of the approved ex‑manufacturer prices, on the day before the reduction day, of any one brand of each of the listed component items, adjusted in accordance with subsection (3).
reduction day component AEMPs means the sum of:
(a) the approved ex‑manufacturer prices, on the reduction day, of any one brand of each of the listed component items, adjusted in accordance with subsection (3); and
(b) if the combination item includes one or more component drugs that are not listed component drugs—the non‑listed component price multiplied by the differential reduction percentage.
(3) For the purposes of the definitions of day before component AEMPs, non‑listed component price and reduction day component AEMPs in subsection (2), adjust the approved ex‑manufacturer price of a brand of a listed component item so that the value attributed to the listed component drug in the combination item reflects:
(a) any difference in quantity or amount; and
(b) any difference in pricing quantity;
of the listed component drug in the listed component item.
(4) For the purposes of paragraph (c) of the definition of listed component item in subsection (2), if there is more than one pharmaceutical item that has the smallest difference as referred to in that paragraph, the pharmaceutical item that:
(a) is not an exempt item; and
(b) results in the smallest reduction under this section to the approved ex‑manufacturer price of the brand of the combination item;
is taken to be the listed component item for the purposes of this section.
2 At the end of Part 7
Add:
Subdivision E—Price reduction
85A Flow on price reductions for brands of combination items
(1) This section sets out, for the purposes of subsection 99ADHB(2) of the Act, the method for calculating the reduced approved ex‑manufacturer price of an existing brand of a combination item on the reduction day mentioned in that subsection.
(2) The reduced approved ex‑manufacturer price of the brand of the combination item is the amount worked out by the following formula:
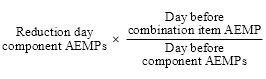
 where:
where:
component drug, in relation to a drug in a combination item, means a drug or medicinal preparation that is contained in that drug.
day before combination item AEMP means the approved ex‑manufacturer price of the brand of the combination item on the day before the reduction day.
day before component AEMPs means the sum of:
(a) the approved ex‑manufacturer prices, on the day before the reduction day, of any one brand of each of the listed component items, adjusted in accordance with subsection (3); and
(b) if the combination item includes one or more component drugs that are not listed component drugs—the non‑listed component price.
differential reduction percentage means:
(a) if there is only one listed component item for which the approved ex‑manufacturer price of any one brand of the listed component item has been reduced under a provision in Division 3B of Part VII of the Act on the reduction day—the difference between 100% and the percentage by which the approved ex‑manufacturer price of any one brand of the listed component item in the combination item has been so reduced; or
(b) if there are 2 or more listed component items for which the approved ex‑manufacturer price of any one brand of each of those listed component items has been reduced under a provision in Division 3B of Part VII of the Act on the reduction day—the difference between 100% and the average of the percentages by which the approved ex‑manufacturer price of any one brand of each of those listed component items has been so reduced.
listed component drug means a component drug in relation to which a declaration under subsection 85(2) is in force.
listed component item, for each listed component drug that is in the combination item and in a non‑combination item as mentioned in paragraph 99ADHB(1)(d) of the Act, means the pharmaceutical item that has:
(a) the same listed component drug as the non‑combination item; and
 (b) the same manner of administration as the combination item as referred to in subsection 99ADHB(7) of the Act; and
(b) the same manner of administration as the combination item as referred to in subsection 99ADHB(7) of the Act; and
(c) subject to subsection (4) of this section, the smallest difference in the total quantity or amount of the listed component drug contained in the quantity or number of units in the pricing quantity of any one brand of the pharmaceutical item compared to the total quantity or amount of the listed component drug in the pricing quantity of the brand of the combination item.
non‑listed component price means the day before combination item AEMP reduced (but not below zero) by the sum of the approved ex‑manufacturer prices, on the day before the reduction day, of any one brand of each of the listed component items, adjusted in accordance with subsection (3).
reduction day component AEMPs means the sum of:
(a) the approved ex‑manufacturer prices, on the reduction day, of any one brand of each of the listed component items, adjusted in accordance with subsection (3); and
(b) if the combination item includes one or more component drugs that are not listed component drugs—the non‑listed component price multiplied by the differential reduction percentage.
(3) For the purposes of the definitions of day before component AEMPs, non‑listed component price and reduction day component AEMPs in subsection (2), adjust the approved ex‑manufacturer price of a brand of a listed component item so that the value attributed to the listed component drug in the combination item reflects:
(a) any difference in quantity or amount; and
(b) any difference in pricing quantity;
of the listed component drug in the listed component item.
(4) For the purposes of paragraph (c) of the definition of listed component item in subsection (2), if there is more than one pharmaceutical item that has the smallest difference as referred to in that paragraph, the pharmaceutical item that:
(a) is not an exempt item; and
(b) results in the smallest reduction under this section to the approved ex‑manufacturer price of the brand of the combination item;
is taken to be the listed component item for the purposes of this section.
3 At the end of Part 9
Add:
103 Application provision relating to the National Health (Pharmaceutical Benefits) Amendment (2021 Measures No. 1) Regulations 2021
Sections 65A and 85A, as inserted by Part 1 of Schedule 1 to the National Health (Pharmaceutical Benefits) Amendment (2021 Measures No. 1) Regulations 2021, apply in relation to reduction days occurring on or after 1 July 2022.
Part 2—Amendments commencing 1 October 2022
National Health (Pharmaceutical Benefits) Regulations 2017
4 Paragraph 84(1)(b)
Repeal the paragraph, substitute:
(b) at the end of the previous data collection period either:
(i) the drug in the WADP brand had been on F2 for at least 18 months and during that period there has been no price reduction under Division 3B of Part VII of the Act to any brand of pharmaceutical item that has the same drug and manner of administration as the WADP brand; or
(ii) the drug in the WADP brand had been on F2 for at least 30 months; and
5 Paragraph 84(1)(c)
Omit “at least 30 months”, substitute “at least as many months as mentioned in subparagraph (b)(i) or (ii) (whichever is applicable)”.
6 Section 103
Before “Sections”, insert “(1)”.
7 At the end of section 103
Add:
(2) The amendments made to section 84 by Part 2 of Schedule 1 to those Regulations apply to data collection periods beginning on or after 1 April 2022.
Part 3—Amendments commencing 1 April 2023
National Health (Pharmaceutical Benefits) Regulations 2017
8 After section 73
Insert:
73A Step 3A—adjusted net revenue for brand
(1) Work out the adjusted net revenue of the listed brand of the pharmaceutical item for the data collection period.
Where average approved ex‑manufacturer price is $4 or less
(2) If the average approved ex‑manufacturer price (see step 3) of the listed brand of the pharmaceutical item for the data collection period for the brand is $4 or less, the adjusted net revenue is the amount worked out by multiplying:
(a) the adjusted volume of the listed brand of the pharmaceutical item sold for the data collection period (see step 2); by
(b) the average approved ex‑manufacturer price of the listed brand of the pharmaceutical item for the data collection period (see step 3).
Example: A responsible person has one listed brand (brand A) of a pharmaceutical item that has an average approved ex‑manufacturer price of $3.50 and the net revenue obtained for a sale of 1,000 packs of that brand was $3,000. The adjusted net revenue of brand A is $3,500.
Where average approved ex‑manufacturer price is more than $4
(3) If the average approved ex‑manufacturer price (see step 3) of the listed brand (the relevant brand) of the pharmaceutical item for the data collection period is more than $4, the adjusted net revenue is the amount worked out by:
(a) working out the sum of the net revenue worked out under step 1 for all listed brands of pharmaceutical items to which both of the following apply for the data collection period:
(i) the responsible person for the brand of the pharmaceutical item is the same as the responsible person for the relevant brand;
(ii) the average approved ex‑manufacturer price of the brand of the pharmaceutical item is $4 or less; and
(b) working out what would have been the sum of the net revenue of all listed brands of pharmaceutical items to which paragraph (a) applies for the data collection period if each of those listed brands had instead been sold for the average approved ex‑manufacturer prices by:
(i) multiplying the adjusted volume worked out under step 2 for each of those listed brands by the average approved ex‑manufacturer price for each of those brands worked out under step 3; and
(ii) adding up the amounts worked out under subparagraph (i); and
(c) reducing (but not below zero) the amount worked out under paragraph (b) by the amount worked out under paragraph (a); and
(d) working out the net revenue adjustment percentage (expressed as a percentage to 2 decimal places) by dividing the amount worked out under paragraph (c) by the sum of the net revenue (worked out under step 1) of all listed brands of pharmaceutical items to which both of the following apply for the data collection period:
(i) the responsible person for the brand of the pharmaceutical item is the same as the responsible person for the relevant brand;
(ii) the average approved ex‑manufacturer price of the brand of pharmaceutical item is more than $4; and
(e) reducing the net revenue for the relevant brand for the data collection period by the net revenue adjustment percentage.
Note: The effect of this subsection is that any difference between net revenue and the revenue that would have been obtained by a responsible person had they supplied brands of pharmaceutical items with approved ex‑manufacturer prices of $4 or less at the average approved ex‑manufacturer prices for those brands will be apportioned to the net revenue for the responsible person’s brands of pharmaceutical items that have approved ex‑manufacturer prices of more than $4.
Example: The responsible person for brand A is also the responsible person for two listed brands of pharmaceutical items that have approved ex‑manufacturer prices of more than $4. The net revenue for one of those brands (brand B) is $2,000 and for the other (brand C) is $3,000. The net revenue adjustment percentage is obtained by dividing $500 (the difference between the adjusted net revenue and the net revenue of brand A) by $5,000 (the sum of the net revenue of brand B and brand C) which equals 10%. The adjusted net revenue of brand B is $1,800 ($2,000 reduced by 10%) and the adjusted net revenue of brand C is $2,700 ($3,000 reduced by 10%).
9 Subparagraph 74(2)(b)(i)
Omit “net revenue for the listed brand (see step 1)”, substitute “adjusted net revenue for the listed brand (see step 3A)”.
10 Subsection 85(2)
Omit “, other than the supply to a public hospital”.
11 After subsection 85(2)
Insert:
(2A) Subsection (2) does not apply to a supply of a brand of a pharmaceutical item during a data collection period for the brand if:
(a) the supply is to a public hospital; and
(b) the drug in the pharmaceutical item had not been on F2 for at least 42 months at the end of the previous data collection period for the brand.
12 At the end of section 103
Add:
(3) Section 73A, as inserted by Part 3 of Schedule 1 to those Regulations, and the amendments made to section 74 by Part 3 of Schedule 1 to those Regulations, apply to data collection periods beginning on or after 1 October 2022.
(4) The amendments made to section 85 by Part 3 of Schedule 1 to those Regulations apply to information in relation to supplies of brands of pharmaceutical items occurring on or after 1 October 2022.
Part 4—Amendments commencing 1 July 2023
National Health (Pharmaceutical Benefits) Regulations 2017
13 Part 7 (heading)
Repeal the heading, substitute:
Part 7—Price reduction, price disclosure and stockholding
14 At the end of Part 7
Add:
Division 3—Stockholding requirements
85B Usual demand for a brand
(1) For the purposes of subsection 99AEKC(5) of the Act, the usual demand for a brand of a pharmaceutical item for a month in a data collection period for that brand is the number of packs of the brand supplied during the data collection period (the reference period) before the previous data collection period for the brand divided by the number of months in the reference period.
(2) For the purposes of subsection (1) the number of packs of the brand supplied during a data collection period is taken to be:
(a) the number provided in accordance with paragraph 85(2)(h) for that brand for that period; and
(b) adjusted as if the size of the pack equals the pricing quantity of the brand.
(3) If a brand of a pharmaceutical item is not a listed brand in the reference period referred to in subsection (1), the usual demand for the brand for the month referred to in that subsection is taken to be zero.
85C Stockholding disclosure requirements
(1) This section is made for the purposes of subsection 99AEKF(1) of the Act.
Prescribed information
(2) The responsible person for a brand of a pharmaceutical item must provide the following information in relation to the quantity of the brand of pharmaceutical item kept in stock in Australia:
(a) the start and end dates of the period to which the information relates;
(b) the name of the brand;
(c) the name of the responsible person;
(d) the name of the drug in the pharmaceutical item;
(e) the form of the drug, including its strength;
(f) the manner of administration of the form of the drug;
(g) the number or quantity of units in a pack (the number of tablets in a pack, for example);
(h) the number of packs held in stock at the end of each month in the period.
Prescribed person
(3) The responsible person must provide the information to the same person to whom the responsible person must give information under subsection 85(6).
Prescribed manner and form
(4) The responsible person must provide the information in a form approved, in writing, by the Secretary.
(5) The completed form must:
(a) include all the statements and information required by the form; and
(b) be signed (or authorised for electronic transmission) by a person who is authorised by the responsible person to provide the information.
Prescribed times
(6) Subject to subsection (7), the responsible person must provide the information:
(a) for each period between 1 April and 30 September in a year—before the end of 11 November in that year; and
(b) for each period between 1 October and the next 31 March—before the end of the next 12 May.
(7) However, for the period between a brand’s start day and the next 31 March or 30 September, whichever is the sooner, the responsible person must provide the information:
(a) if the start day happens between 1 April and 30 September in a year—before the end of 11 November in that year; or
(b) if the start day happens between 1 October and the next 31 March—before the end of the next 12 May.
15 At the end of section 103
Add:
(5) Sections 85B and 85C, as inserted by Part 4 of Schedule 1 to those Regulations, apply to information in relation to supplies of brands of pharmaceutical items occurring on or after 1 July 2023.