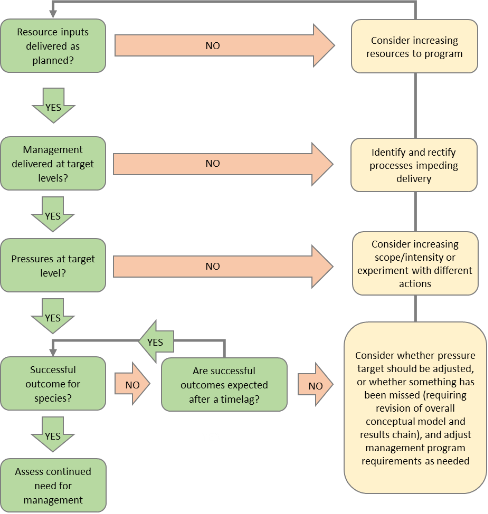
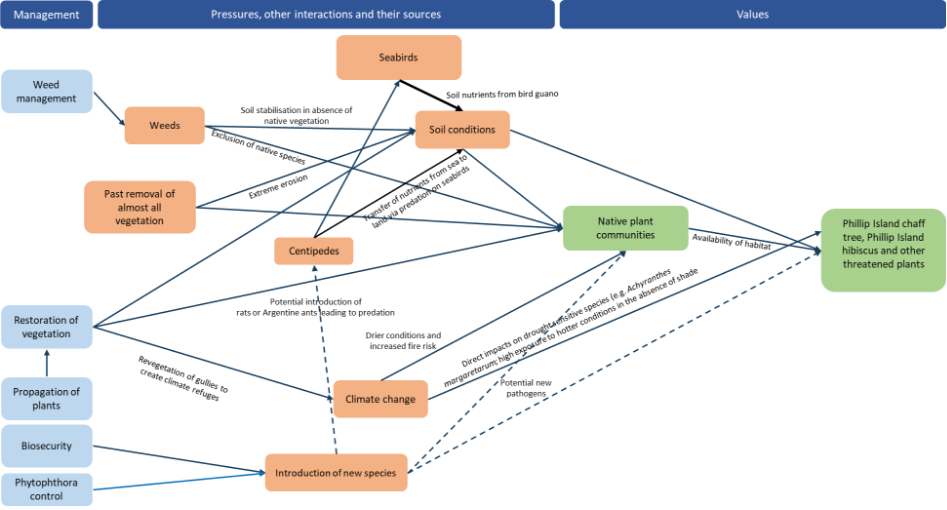
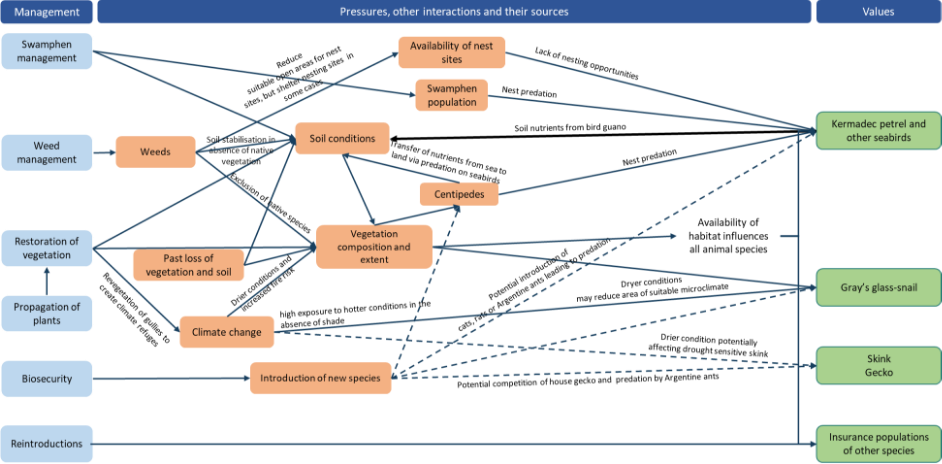
During the first year of the plan significant work will be needed to set up coordination and governance arrangements, including: the coordination group/recovery team; a plan for monitoring, evaluation of management effectiveness and reporting; and a citizen science and volunteer program.
There will be a need to prioritise activities, taking into consideration available resources and any new information. Priorities should be regularly reviewed and revised over the life of the plan.
In the first two years of the plan, a series of operational and strategic plans for delivery of management programs should be developed, including any research that is needed to inform these strategies. Work should also begin on investigating potential legislative changes (for example, to better protect native vegetation).
Once operational, most management programs will require ongoing action and be regularly reviewed and adjusted. The intensity of some programs may need to be increased over the 10 years of the program based on the results of evaluation in the initial years.
The timing of species-specific interventions (such as possible captive breeding and translocations) will be dependent on feasibility studies and decision analysis, and on data still to be collected and decisions yet to be taken.
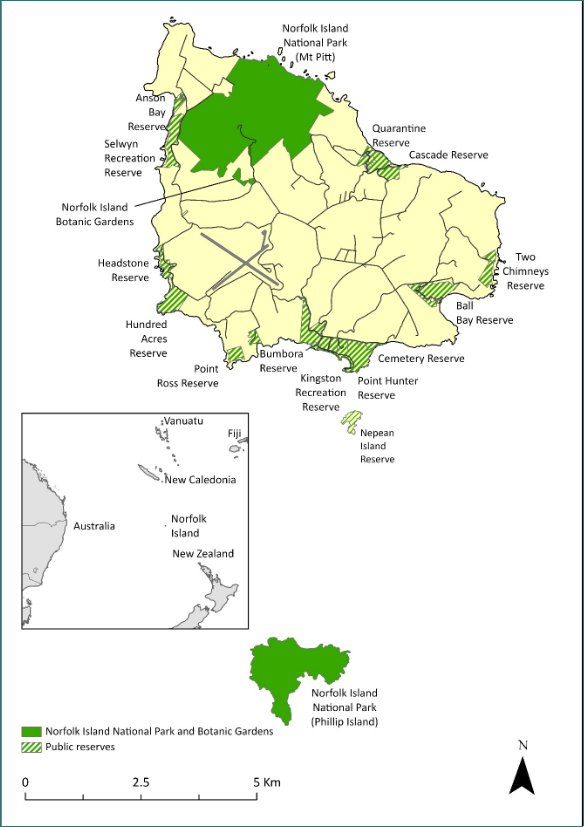
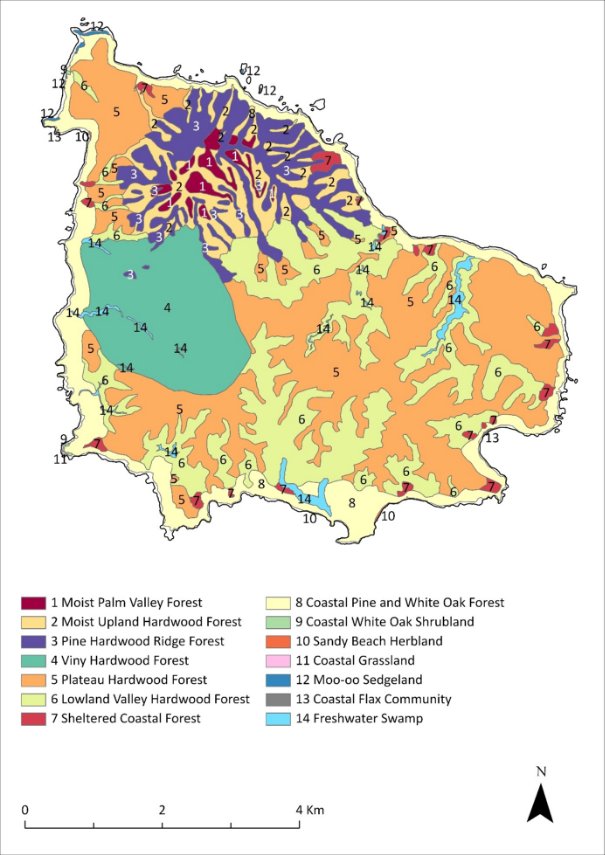
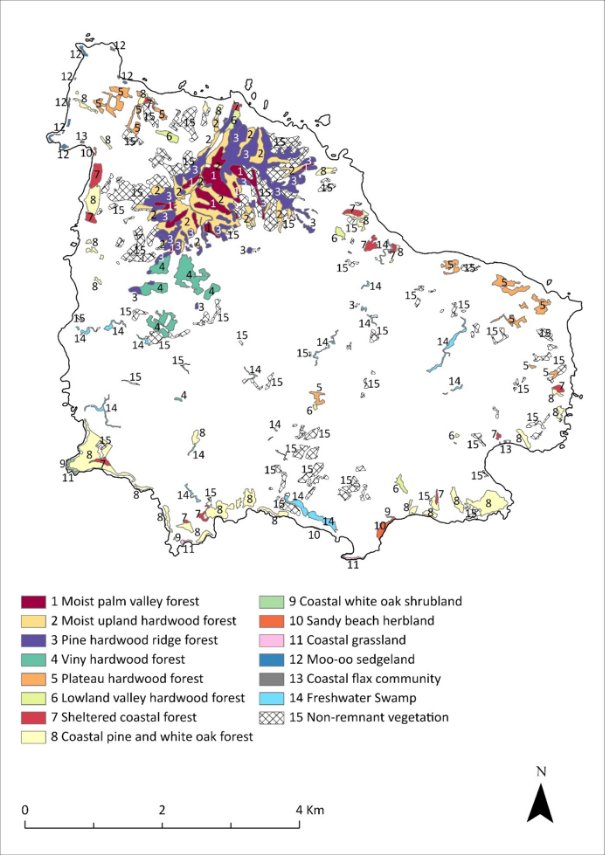
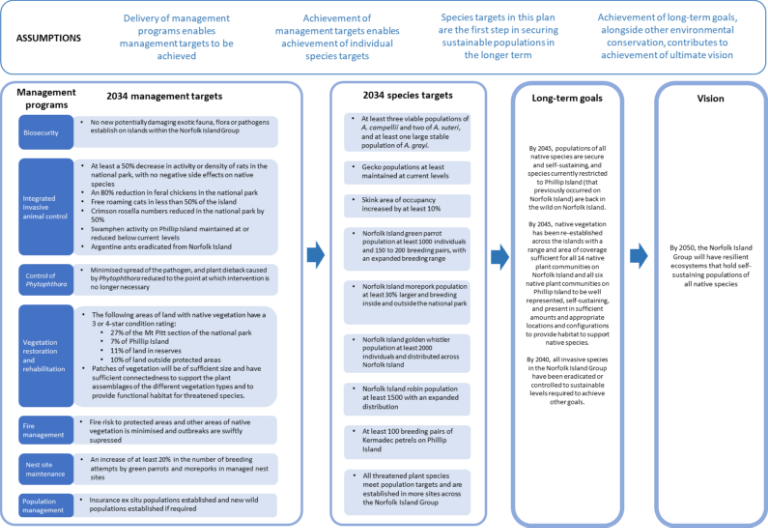
While this recovery plan is made for a specific subset of listed threatened species in the Norfolk Island Group, profiles have been included for each of the threatened species, as well as the region’s most significant seabirds, some of which are listed as marine and/or migratory under the EPBC Act. Distribution maps are included for the extant listed threatened species only, and only cover their Norfolk Island Group range. Risks assessments have been undertaken for the threatened species and a small number of additional species: the Norfolk Island stag beetle (listed on the IUCN red list though not under the EPBC Act), the slender-billed white eye and several priority seabirds (white-necked petrel, providence petrel and sooty tern).
6.1 Invertebrates..........................................................155
Advena campbellii—Campbell’s keeled glass-snail................................155
Advena grayi—Gray’s glass-snail............................................159
Advena phillipii—Phillip Island glass-snail......................................162
Advena stoddartii—Stoddart’s glass-snail......................................164
Advena suteri—Suter’s striped glass-snail......................................166
Lamprima aenea—Norfolk Island stag beetle/Norfolk Island Christmas beetle...........170
6.2 Reptiles..............................................................172
Christinus guentheri—Lord Howe Island gecko..................................172
Oligosoma lichenigerum—Lord Howe Island skink................................176
6.3 Land birds.............................................................180
Cyanoramphus cookii—Norfolk Island green parrot...............................180
Ninox novaeseelandiae undulata—Norfolk Island morepork........................185
Pachycephala pectoralis xanthroprocta—Norfolk Island golden whistler (tamey).........190
Petroica multicolor—Norfolk Island robin......................................194
Zosterops albogularis—white-breasted white-eye, grinnell.........................198
Zosterops tenuirostris—slender-billed white-eye.................................200
6.4 Seabirds..............................................................202
Anous albivittus albivittus—Tasman grey noddy, grey ternlet (western pacific)...........202
Anous minutus—black noddy...............................................204
Anous stolidus—common noddy............................................206
Ardenna carneipes—flesh-footed shearwater...................................207
Ardenna pacifica—wedge-tailed shearwater, ghost bird...........................209
Fregetta grallaria grallaria—Tasman white-bellied storm-petrel.....................211
Gygis alba—white tern...................................................212
Morus serrator—Australasian gannet.........................................213
Onychoprion fuscata—sooty tern, whale bird...................................215
Phaethon rubricauda—red-tailed tropicbird....................................218
Pterodroma cervicalis—white-necked petrel....................................220
Pterodroma neglecta neglecta—Kermadec petrel (western)........................224
Pterodroma nigripennis—black-winged petrel...................................229
Pterodroma solandri—providence petrel......................................231
Puffinus assimilis—little shearwater..........................................235
Sula dactylatra—masked booby.............................................237
6.5 Flora.................................................................239
Abutilon julianae—Norfolk Island abutilon.....................................239
Achyranthes arborescens—chaff tree, soft-wood.................................242
Achyranthes margaretarum—Phillip Island chaffy tree............................246
Anthosachne kingiana kingiana—Phillip Island wheat-grass.........................249
Blechnum norfolkianum—Norfolk Island water-fern..............................253
Boehmeria australis australis—tree nettle, nettletree.............................257
Calystegia affinis—a creeper...............................................260
Clematis dubia—clematis..................................................263
Coprosma baueri—coastal coprosma.........................................266
Coprosma pilosa—mountain coprosma........................................270
Cordyline obtecta—Ti....................................................273
Dendrobium brachypus—Norfolk Island orchid..................................276
Dysoxylum bijugum—sharkwood............................................279
Elatostema montanum—mountain procris.....................................282
Euphorbia norfolkiana—Norfolk Island euphorbia................................285
Euphorbia obliqua—a herb................................................289
Hibiscus insularis—Phillip Island hibiscus......................................292
Hypolepis dicksonioides—downy ground fern, brake fern...........................295
Ileostylus micranthus—mistletoe............................................298
Lastreopsis calantha—shield-fern............................................301
Marattia salicina (Ptisana salicina)—king fern, para, potato fern.....................304
Melicope littoralis—shade tree..............................................307
Melicytus latifolius—Norfolk Island mahoe.....................................310
Melicytus ramiflorus subsp. oblongifolius—whiteywood...........................313
Meryta angustifolia—Narrow-leaved Meryta...................................316
Meryta latifolia—broad-leaved meryta........................................319
Muehlenbeckia australis—shrubby creeper, pohuehue............................323
Myoporum obscurum—popwood............................................326
Myrsine ralstoniae—beech................................................330
Pennantia endlicheri—pennantia............................................333
Phreatia limenophylax—Norfolk Island phreatia.................................337
Phreatia paleata—White lace orchid.........................................340
Pittosporum bracteolatum—oleander.........................................343
Planchonella costata—bastard ironwood......................................346
Polyphlebium endlicherianum—middle filmy fern................................349
Pteris kingiana—King’s brakefern............................................352
Pteris zahlbruckneriana—netted brakefern.....................................356
Senecio australis—a daisy.................................................359
Senecio evansianus—a daisy...............................................363
Senecio hooglandii—a daisy................................................366
Streblus pendulinus—Siah’s backbone........................................369
Taeniophyllum norfolkianum—minute orchid, ribbon‑root orchid..............373
Tmesipteris norfolkensis—hanging fork-fern....................................376
Ungeria floribunda—bastard oak............................................379
Wikstroemia australis—kurrajong...........................................382
Zehneria baueriana—native cucumber, giant cucumber...........................386
Advena campbellii—Campbell’s keeled glass-snail
Conservation significance
Endemic to the Norfolk Island Group.
EPBC Act Listing Status: Critically Endangered
Non-statutory Listing Status: Listed as Extinct on the IUCN Red List (IUCN 2020)
Approved Conservation Advice: 19/12/2008 (DEWHA 2008a).
Description
A small land snail, which usually has a bi-coloured shell with an elevated fawn spire and a black round base. Typical specimen is about 17 mm in diameter and 11 mm high.
Distribution
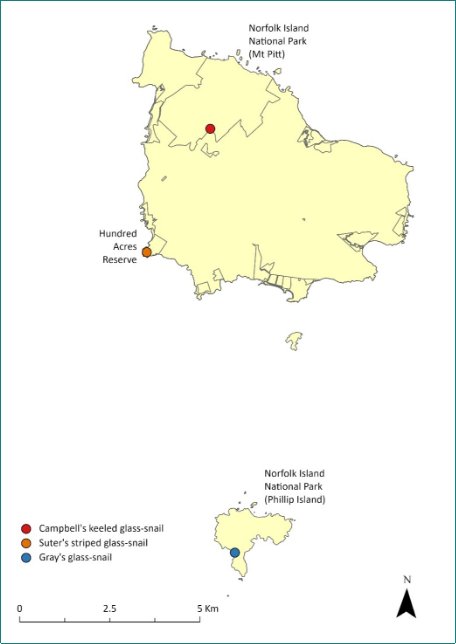
Campbell’s keeled glass-snail (Advena campbellii) was once common on Phillip Island and pre-European fossil records suggest it was once abundant in the Emily Bay-Cemetery Bay area of Norfolk Island (Varman 1991; Neuweger et al. 2001).
Its range contraction and rarity suggested it may be close to extinction, with the species only being recorded in the national park and botanic garden by 2008 (DEWHA 2008).
In surveys undertaken in March 2020 (Hyman & Kohler 2020), Campbell’s keeled glass-snail was observed east of Mt Pitt Road and near the national park boundary, both inside and outside the park. Twenty-one living specimens and over 40 empty shells were observed over a 1.5 person-hour search in an area approximately 10 x 60 m. In subsequent surveys in October 2020 and May 2021 the population size had increased, as had the size of the area in which they were found. The current estimated population size is 500 individuals, based on a count of 197 live specimens in May 2022 and 137 live specimens in November 2022, distributed over 1.3 ha and three populations. The distribution is shown in Map 13.
Ecology
Live bearing and the largest of the native land snails.
Habitat
It is known to live under leaf litter, logs and rocks; particularly common under fallen palm fronds (Smith 1992, Hyman & Kohler 2020).
Threats
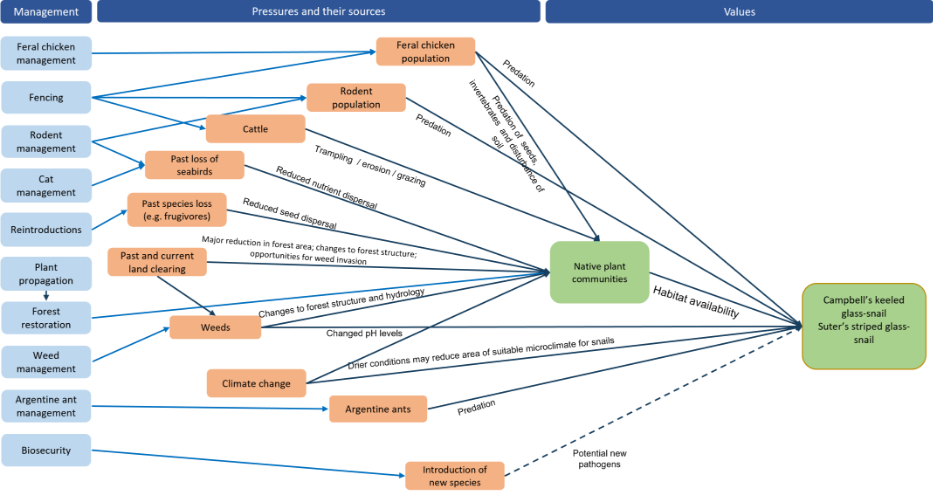
Major threats include habitat loss, fragmentation and degradation by land clearing and stock grazing, as well as the introduction of feral animals (notably rats) and invasive weeds. Predation by rodents and feral chickens remains a significant threat. Many empty shells found in the 2020 survey showed clear signs of rodent predation, and there were also signs of feral chickens in the area. Drying conditions and lower soil moisture balances due to climate change are also a threat.
Map 13 Distribution of Advena campbellii
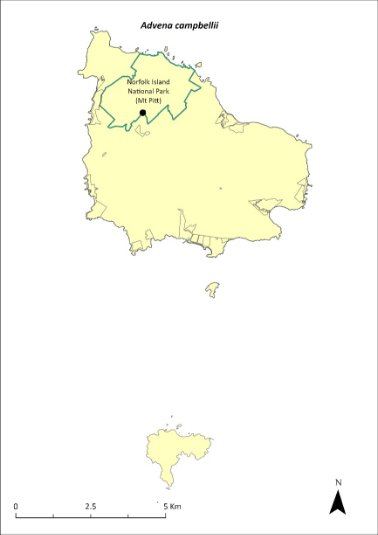
Green outlines indicate reserves within which the species occurs. Points show recorded locations (Hyman & Kohler 2020).
Impact on other species
None known.
Risk assessment
The risk assessment is shown in Table 36.
Table 36 Risk assessment for Advena campbellii
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Major | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Unlikely (11–25%) | Major | Medium |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Moderate | High |
4. Degradation of native vegetation through current or future grazing | Unlikely (11–25%) | Moderate | Low |
6. Predation by rodents | Almost certain (91–100%) | Extreme | Extreme |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Likely (51–90%) | Major | High |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Possible (26–50%) | Minor | Low |
12. Infection by pathogens already present | Unlikely (11–25%) | Minor | Low |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Moderate | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Likely (51–90%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Likely (51–90%) | Moderate | Medium |
Management actions
Restore native vegetation, control introduced weeds and feral animals (primarily rodents and chickens). Reduce predation pressure by targeting control of rodents in areas where there are known snail populations. Remove weeds (especially red guava) from important areas for snails to restore suitable pH and moisture levels. Conduct further surveys to determine the full extent of existing populations.
Continue the captive breeding program at Taronga Zoo and return the species to appropriate managed sites on Norfolk Island (ensuring exclusion of rodents and chickens).
Recovery target
The recovery target is shown in Table 37.
Table 37 Recovery target for Advena campbellii
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Critically Endangered | 500 (3 populations) | 90% in national park 10% outside the park and reserves | At least three viable populations maintained on Norfolk Island |
Relevant literature
DEWHA (Department of the Environment, Water, Heritage and the Arts) (2008a) Approved Conservation Advice for Advena campbellii campbellii. Department of the Environment, Water, Heritage and the Arts, Canberra.
Hyman I & Köhler F (2020) Report on survey of land snails on Norfolk Island. Australian Museum, Sydney.
Hyman I (2005) Taxonomy, systematic, and evolutionary trends in Helicarionida (Mollusca, Pulmonata). PhD Thesis, University of Sydney.
Hyman IT, Caiza J & Köhler F (2023) Systematic revision of the microcystid land snails endemic to Norfolk Island (Gastropoda: Stylommatophora) based on comparative morpho-anatomy and mitochondrial phylogenetics. Invertebrate Systematics 37(5–6), 334–443.
Iredale T (1945) The land mollusca of Norfolk Island. Australian Zoologist 11, 46–71.
IUCN (2020) 2020 IUCN Red List of Threatened Species. Accessed 23 January 2024.
Neuweger D, White P & Ponder WF (2001) Land snails from Norfolk Island sites. Records of the Australian Museum Supplement 27, 115–122.
Ponder WF (1997) Conservation status, threats and habitat requirements of Australian terrestrial and freshwater mollusca. Memoirs of the Museum of Victoria 56, 421–430.
Smith BJ (1992) Non-marine Mollusca, in WWK Houston (ed) Zoological Catalogue of Australia Volume 8. Australian Government Publishing Service, Canberra.
Varman RVJP (1991) Conchological Survey 1983-90: Manuscript of Land Mollusca Fossiliferous and Present Day. Unpublished manuscript.
Advena grayi—Gray’s glass-snail
Conservation significance
Endemic to the Norfolk Island Group.
EPBC Act Listing Status: Critically Endangered (listed as Mathewsoconcha grayi ms).
Approved Conservation Advice: 19/12/2008 (DEWHA 2008b).
Description
This species has a similar shell to Advena suteri but the spire is slightly higher and the shell distinctly larger and more inflated. Typical specimen is 15 mm in diameter and 11 mm in height (Hyman 2005). There is no peripheral band.
Distribution
Fossils of this species were found on Nepean Island, and it was common in sub-fossil deposits on Norfolk Island but was not located in native forests during surveys between 1983 and 1990 (Varman 1991). The only previous non-fossil material for this species came from two specimens collected on Phillip Island in 1982 (TSSC 2009b).
The species was thought to be extinct on both Norfolk Island and Phillip Island but was recently rediscovered surviving in flax on slopes on Phillip Island. Based on a survey in March 2023, the estimated (conservative approximated) population size is 5,000, with one population over an area of 0.93ha. The population may be very weather dependant and go through boom-and-bust cycles.
The distribution is shown in Map 14.
Ecology
Live-bearing.
Habitat
Litter and woodland (Smith 1992).
Threats
Major threats include habitat loss, fragmentation and degradation by land clearing and stock grazing, as well as the introduction of feral animals (notably rats) and invasive weeds. Predation by rodents and feral chickens remains a significant threat. Drying conditions and lower soil moisture balances due to climate change are also a threat.
Impact on other species
None known.
Map 14 Distribution of Advena grayi
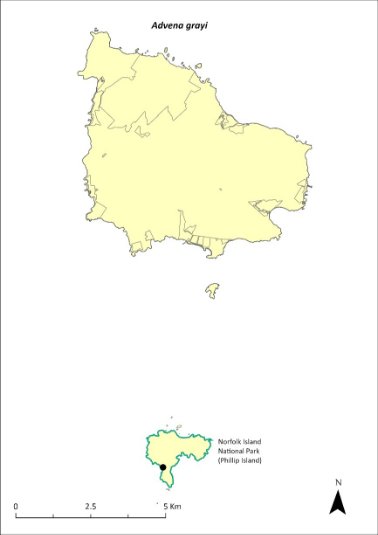
Green outlines indicate reserves within which the species occurs. Points show recorded locations (Tweed 2023).
Risk assessment
The risk assessment is shown in Table 38.
Table 38 Risk assessment for Advena grayi
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Major | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Unlikely (11–25%) | Major | Medium |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Moderate | High |
4. Degradation of native vegetation through current or future grazing | Unlikely (11–25%) | Moderate | Low |
6. Predation by rodents | Rare (0–10%) | Extreme | Medium |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Major | Low |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Almost certain (91–100%) | Minor | Medium |
12. Infection by pathogens already present | Unlikely (11–25%) | Minor | Low |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Moderate | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Likely (51–90%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Possible (26–50%) | Moderate | Medium |
Management actions
Restore native forest habitat, control introduced weeds and predators (rodents, chickens), survey to determine the extent of existing populations and consider captive breeding.
Recovery target
The recovery target is shown in Table 39.
Table 39 Recovery target for Advena grayi
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Critically Endangered | 5,000 (1 population) | 100% within the national park | At least one large population on Phillip Island |
Relevant literature
DEWHA (Department of the Environment, Water, Heritage and the Arts) (2008b) Approved Conservation Advice for Mathewsoconcha grayi ms (a snail). Department of the Environment, Water, Heritage and the Arts, Canberra.
Hyman I (2005) Taxonomy, systematic, and evolutionary trends in Helicarionida (Mollusca, Pulmonata). PhD Thesis, University of Sydney.
Hyman IT, Caiza J & Köhler F (2023) Systematic revision of the microcystid land snails endemic to Norfolk Island (Gastropoda: Stylommatophora) based on comparative morpho-anatomy and mitochondrial phylogenetics. Invertebrate Systematics 37(5–6), 334–443.
Smith BJ (1992) Non-marine Mollusca, in WWK Houston (ed) Zoological Catalogue of Australia Volume 8. Australian Government Publishing Service, Canberra.
TSSC (Threatened Species Scientific Committee) (2009b) Commonwealth Listing Advice on Mathewsoconcha grayi ms. Department of the Environment, Water, Heritage and the Arts, Canberra.
Tweed J (2023) Phillip Island Survey March 2023. Unpublished data.
Varman RVJP (1991) Conchological Survey 1983-90: Manuscript of Land Mollusca Fossiliferous and Present Day. Unpublished manuscript.
Advena phillipii—Phillip Island glass-snail
Conservation significance
Endemic to the Norfolk Island Group.
EPBC Act Listing Status: Critically Endangered (listed as Mathewsoconcha phillipii).
Approved Conservation Advice: 19/12/2008 (DEWHA 2008c).
Description
Very similar to Advena grayi but has a slightly larger shell, the spire is shorter and there is a white narrow peripheral band. Typical specimen is 16 mm in diameter and 12 mm in height (Hyman 2005).
Distribution
This species is known from non-fossil material only from two official specimens collected from Phillip Island in 1908 (TSSC 2009c). Fossils of this species were collected from the Cemetery Bay area of Norfolk Island (Varman 1991). It is likely extinct on Norfolk Island and restricted to Phillip Island where, because of the previous destruction of the vegetation by rabbits, it is unlikely to be anything other than very rare.
Ecology
Live-bearing.
Habitat
Saxicoline, under rocks (Smith 1992).
Threats
Major threats include habitat loss, fragmentation and degradation by land clearing and stock grazing, as well as the introduction of feral animals (notably rats) and invasive weeds. Drying conditions and lower soil moisture balances due to climate change remain a threat to endemic snails in the Norfolk Island Group.
Impact on other species
None known.
Risk assessment
Not undertaken as species is presumed extinct.
Management actions
Restore native forest habitat, control introduced weeds and predators (chickens, rodents), survey to determine the presence of any existing populations and if found to be extant, consider captive breeding.
Recovery target
Not applicable as species is presumed extinct.
Relevant literature
DEWHA (Department of the Environment, Water, Heritage and the Arts) (2008c) Approved Conservation Advice for Mathewsoconcha phillipii (Phillip Island Helicarinoid Snail). Department of the Environment, Water, Heritage and the Arts, Canberra.
Hyman I (2005) Taxonomy, systematic, and evolutionary trends in Helicarionida (Mollusca, Pulmonata). PhD Thesis, University of Sydney.
Hyman IT, Caiza J & Köhler F (2023) Systematic revision of the microcystid land snails endemic to Norfolk Island (Gastropoda: Stylommatophora) based on comparative morpho-anatomy and mitochondrial phylogenetics. Invertebrate Systematics 37(5–6), 334–443.
Iredale T (1945) The land mollusca of Norfolk Island. Australian Zoologist 11, 46–71.
Ponder WF (1997) Conservation status, threats and habitat requirements of Australian terrestrial and freshwater mollusca. Memoirs of the Museum of Victoria 56, 421–430.
Smith BJ (1992) Non-marine Mollusca, in WWK Houston (ed) Zoological Catalogue of Australia Volume 8. Australian Government Publishing Service, Canberra.
TSSC (Threatened Species Scientific Committee) (2009c) Commonwealth Listing Advice on Mathewsoconcha phillipii. Department of the Environment, Water, Heritage and the Arts, Canberra.
Varman RVJP (1991) Conchological Survey 1983-90: Manuscript of Land Mollusca Fossiliferous and Present Day. Unpublished manuscript.
Advena stoddartii—Stoddart’s glass-snail
Conservation significance
Endemic to the Norfolk Island Group.
EPBC Act Listing Status: Critically Endangered (listed as Quintalia stoddartii).
Non-statutory Listing Status: Listed as Extinct on the IUCN Red List (IUCN 2020).
Approved Conservation Advice: 19/12/2008 (DEWHA 2008e).
Description
The species has an imperforate, depressed conical shell with dimensions of at least 14mm diameter and 8mm height.
Distribution
Early records and sub-fossil material suggest this species once occurred on all three islands. In the early 1900s this species made up as much as 9% of total snail specimens collected on Norfolk Island. However, recent surveys have failed to locate this species; it was last collected at Ball Bay and Duncombe Bay in 1945, and is likely extinct on Norfolk Island.
No museum-held specimens from Phillip Island exist apart from the type material that was collected in 1834 (Hyman 2005). A specimen that is currently held in a private collection was collected from Phillip Island in the 1990s, indicating that this species may have survived. While no specimens were found in a single recent targeted survey, the lack of sampling from Phillip Island provides hope that it may still be extant there.
Ecology
Live-bearing.
Habitat
Saxicoline, under rocks (Smith 1992).
Threats
Major threats include habitat loss, fragmentation and degradation by land clearing and stock grazing, as well as the introduction of feral animals (notably rats) and invasive weeds. Drying conditions and lower soil moisture balances due to climate change remain a threat to endemic snails in the Norfolk Island Group.
Impact on other species
None known.
Risk assessment
Not undertaken as species is presumed extinct.
Management actions
Restore native forest habitat, control introduced weeds and predators (chickens, rodents), survey to determine the presence of any existing populations and if found to be extant, consider captive breeding.
Recovery target
Not applicable as species is presumed extinct.
Relevant literature
DEWHA (Department of the Environment, Water, Heritage and the Arts) (2008e) Approved Conservation Advice for Quintalia stoddartii (Stoddart's Helicarionid Land Snail). Department of the Environment, Water, Heritage and the Arts, Canberra.
Hyman I (2005) Taxonomy, systematic, and evolutionary trends in Helicarionida (Mollusca, Pulmonata). PhD Thesis, University of Sydney.
Hyman IT, Caiza J & Köhler F (2023) Systematic revision of the microcystid land snails endemic to Norfolk Island (Gastropoda: Stylommatophora) based on comparative morpho-anatomy and mitochondrial phylogenetics. Invertebrate Systematics 37(5–6), 334–443.
Iredale T (1945) The land mollusca of Norfolk Island. Australian Zoologist 11, 46–71.
IUCN (2020) 2020 IUCN Red List of Threatened Species. Accessed 24 January 2024.
Ponder WF (1997) Conservation status, threats and habitat requirements of Australian terrestrial and freshwater mollusca. Memoirs of the Museum of Victoria 56, 421–430.
Smith BJ (1992) Non-marine Mollusca, in WWK Houston (ed) Zoological Catalogue of Australia Volume 8. Australian Government Publishing Service, Canberra.
TSSC (Threatened Species Scientific Committee) (2009e) Commonwealth Listing Advice on Quintalia stoddartii. Department of the Environment, Water, Heritage and the Arts, Canberra.
Advena suteri—Suter’s striped glass-snail
Conservation significance
Endemic to the Norfolk Island Group.
EPBC Act Listing Status: Critically Endangered (listed as Mathewsoconcha suteri).
Approved Conservation Advice: 19/12/2008 (DEWHA 2008d).
Description
Suter’s striped glass-snail (Advena suteri) has an orange-brown to fawn shell with a narrow white peripheral band, a depressed spire, and is 9 to 10 mm in diameter and 6 to 6.5 mm high.
Distribution
Archaeological deposits suggest this species was once common but by 1914 it was considered rare (Iredale 1945; Varman 1991). More recent records suggested it was restricted to isolated localities including Norfolk Island National Park in the area around Mt Pitt and Hundred Acres Reserve (TSSC 2009d). By the late 1990s it had appeared to be extinct in the national park (Varman 2015, 2016).
In March 2020, Suter’s striped glass-snail was observed in Hundred Acre Reserve where more than 50 freshly dead shells were observed, but in a period of approximately 6 person-hours of searching, only a single live specimen was found. None of the dead shells were rodent-predated, but the whole area was extremely dry and it is likely that there had been a recent high mortality rate linked to the dry weather (Hyman and Kohler 2020). In May 2021, after more favourable weather, approximately 18 person-hours of searching revealed 52 live specimens, indicating that the population was recovering from the dry period. Based on 2022 surveys, the population size has now grown to 350 individuals over an area of 0.7ha.
The distribution is shown in Map 15.
Ecology
Live-bearing.
Habitat
Litter and woodland (Smith 1992), living under logs.
Threats
Major threats include habitat loss, fragmentation and degradation by land clearing and stock grazing, as well as the introduction of feral animals (notably rats) and invasive weeds. Drying conditions and lower soil moisture balances due to climate change are also a threat.
Impact on other species
None known.
Map 15 Distribution of Advena suteri
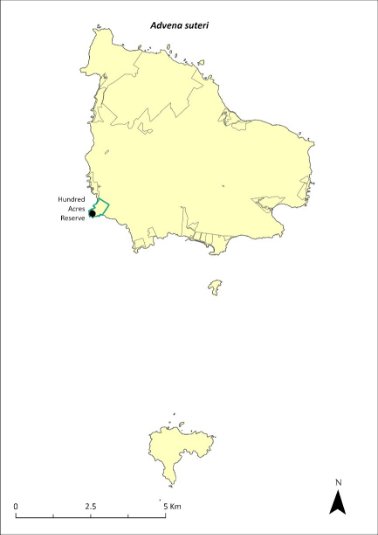
Green outlines indicate reserves within which the species occurs. Points show recorded locations (Hyman & Kohler 2020).
Risk assessment
The risk assessment is shown in Table 40.
Table 40 Risk assessment for Advena suteri
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Major | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Unlikely (11–25%) | Major | Medium |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Moderate | High |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Likely (51–90%) | Extreme | Extreme |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Almost certain (91–100%) | Major | Extreme |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Almost certain (91–100%) | Minor | Medium |
12. Infection by pathogens already present | Unlikely (11–25%) | Minor | Low |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Moderate | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Likely (51–90%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Likely (51–90%) | Moderate | Medium |
Management actions
Restore native vegetation, control introduced weeds and animals (chickens, rodents). Target control of rodents in areas where there are known snail populations to reduce predation pressure. Remove weeds (especially red guava) from important areas for snails to restore suitable pH and moisture levels. Expansion of suitable native vegetation and experimental addition of woody debris to increase the number of shelter sites for the species. Conduct further surveys to determine the full extent of existing populations.
If warranted, reinitiate captive breeding and return individuals to appropriate managed sites on Norfolk Island (ensuring exclusion of rodents and chickens).
Recovery target
The recovery target is shown in Table 41.
Table 41 Recovery target for Advena suteri
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Critically Endangered | 350 (1 population) | 100% within public reserves | Current population maintained and at least one additional viable population established on Norfolk Island |
Relevant literature
DEWHA (Department of the Environment, Water, Heritage and the Arts) (2008d). Approved Conservation Advice for Mathewsoconcha suteri (a snail). Department of the Environment, Water, Heritage and the Arts, Canberra. Accessed 1 February 2024.
Hyman I (2005) Taxonomy, systematic, and evolutionary trends in Helicarionida (Mollusca, Pulmonata). PhD Thesis, University of Sydney.
Hyman I & Köhler F (2020) Report on survey of land snails on Norfolk Island. Australian Museum, Sydney.
Hyman IT, Caiza J & Köhler F (2023) Systematic revision of the microcystid land snails endemic to Norfolk Island (Gastropoda: Stylommatophora) based on comparative morpho-anatomy and mitochondrial phylogenetics. Invertebrate Systematics 37(5–6), 334–443.
Iredale T (1945) The land mollusca of Norfolk Island. Australian Zoologist 11, 46–71.
Ponder WF (1997) Conservation status, threats and habitat requirements of Australian terrestrial and freshwater mollusca. Memoirs of the Museum of Victoria 56, 421–430.
Smith BJ (1992) Non-marine Mollusca, in WWK Houston (ed) Zoological Catalogue of Australia Volume 8. Australian Government Publishing Service, Canberra.
TSSC (Threatened Species Scientific Committee) (2009d) Commonwealth Listing Advice on Mathewsoconcha suteri. Department of the Environment, Water, Heritage and the Arts, Canberra.
Varman RVJ (2015) Norfolk Island Snail Species Collections made between January and March 2015. Unpublished report.
Varman RVJ (2016) Norfolk Island Snail Species Collections made between January and March 2016. Unpublished report.
Varman RVJP (1991) Conchological Survey 1983-90: Manuscript of Land Mollusca Fossiliferous and Present Day. Unpublished manuscript.
Lamprima aenea—Norfolk Island stag beetle/Norfolk Island Christmas beetle
Conservation significance
Endemic to the Norfolk Island Group.
EPBC Act Listing Status: not listed.
Non-statutory Listing Status: Listed as vulnerable on the IUCN Red List (IUCN 2020).
Description
A large metallic-coloured beetle. Males are typically a bright metallic green with large jaws used for fighting other males and typically measure 26–30 mm long. Females are typically smaller (23–27 mm) and have a bronzish tinge (Reid et al. 2018).
Distribution
Very little is known about the distribution of the Norfolk Island stag beetle (Lamprima aenea). During a recent revision of the genus Lamprima, Reid et al. (2018) reported only 10 specimens amongst the material they examined, only one of which had a specified collection location. However, given the widespread distribution of Lord Howe stag beetle (L. insularis) on Lord Howe Island (Reid et al. 2018), it seems likely that the Norfolk Island stag beetle would have been found across the entirety of Norfolk Island, and possibly on Phillip Island prior to its habitat degradation from the impact of introduced species.
Recent records have all been from within the Mt Pitt section of Norfolk Island National Park, though some unconfirmed records suggest it may also still occur in other areas of the island (J Tweed 2024. pers comm 17 January).
Ecology
Little is known of the specific ecology of the Norfolk Island stag beetle; however, it is assumed that the ecology is like that of its close relative the Lord Howe stag beetle on Lord Howe Island (Reid et al. 2018). The larvae develop in rotting wood infected by white-rot fungi and are unlikely to be reliant on a single host tree species.
Habitat
Native forest. Dependent on dead wood for reproduction.
Threats
Major threats include habitat loss, fragmentation and degradation by land clearing and stock grazing, as well as the introduction of feral animals (notably rats and chickens) and invasive weeds. Elytra (modified forewing) showing evidence of rodent predation have been collected, and remains of other large beetle species are frequently found within rat nests in rotting logs within the national park (J Tweed 2024. pers comm 17 January). Drying conditions and lower soil moisture balances due to climate change are also a threat, particularly in its early developmental stages which rely on moist decaying wood. Poaching is also a serious threat to the species as rare stag beetles are highly prized by collectors.
Impact on other species
None known.
Risk assessment
The risk assessment is shown in Table 42.
Table 42 Risk assessment for Lamprima aenea
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Major | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Possible (26–50%) | Major | High |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Moderate | Medium |
4. Degradation of native vegetation through current or future grazing | Unlikely (11–25%) | Moderate | Low |
6. Predation by rodents | Almost certain (91–100%) | Extreme | Extreme |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation by chickens | Likely (51–90%) | Moderate | High |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Moderate | Medium |
11. Competition from/change of habitat because of weed invasion | Possible (26–50%) | Minor | Low |
12. Infection by pathogens already present | Unlikely (11–25%) | Minor | Low |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Likely (51–90%) | Major | High |
15. Problems caused by small populations, including lack of genetic diversity | Likely (51–90%) | Major | High |
Management actions
Restore native vegetation, control introduced weeds and feral animals (rodents, chickens, Argentine ants). Ensure rodent control targets all three known rodent species. Remove and replace red guava and other weeds with native vegetation to provide suitable food plants for larvae. Ensure wind falls and felled trees are left to rot naturally (not burnt or mulched) to provide habitat for reproduction. Conduct further surveys to determine the distribution of the species and improve the understanding of its ecology. Vigilance is required to ensure poachers do not impact the species.
Relevant literature
IUCN (2020) 2020 IUCN Red List of Threatened Species. Accessed 24 January 2024.
Reid AM, Smith K and Beatson M (2018) Revision of the genus Lamprima Latreille, 1804 (Coleoptera: Lucanidae). Zootaxa 4446: 151–202.
Tweed J (2024) Personal communication by email, 17 January. University of Queensland.
Christinus guentheri—Lord Howe Island gecko
Conservation significance
Endemic to the Norfolk Island Group and the Lord Howe Island Group.
EPBC Act Listing Status: Vulnerable.
State Listing Status: Listed as vulnerable under the Biodiversity Conservation Act 2016 (NSW).
Non-statutory Listing Status: Listed as Vulnerable under The Action Plan for Australian Lizards and Snakes 2017 (Chapple et al. 2019).
For further information on the species outside of the Norfolk Island Group, see the species profile on SPRAT.
Distribution
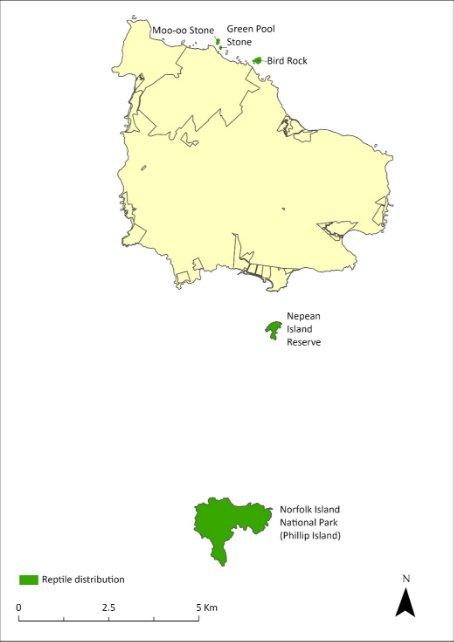
The Lord Howe Island gecko (Christinus guentheri) was described from Norfolk Island and Lord Howe Island in 1885. On the Lord Howe Island complex this species was abundant on the main island until the 1930s, after which it declined dramatically. It is now found only in small numbers in few locations, but it has remained common on some of the other islands of the group, occurring on most vegetated rocky outcrops in the Lord Howe complex.
On the Norfolk Island Group, this species has been found on Nepean and Phillip Islands and on three small rocky islets—Moo-oo Stone, Bird Rock and Green Pool Stone, each about 100 m from the northern cliffs of Norfolk Island. It almost certainly occurs on other rocky islets but it has not been found on the main island and probably became extinct there prior to European settlement (Cogger et al. 2006). Early European reports expressed surprise at the absence of reptiles on the main island; however, remains of this species have been identified on the main island from deposits dating back to 6,500 BP (Cogger et al. 1979).
Cogger et al. (1979) suggested that a conservative estimate for the population on Phillip Island would be 100,000 individuals. The subsequent removal of rabbits and recovery of vegetation on Phillip Island has provided additional suitable habitat for this species. A 2005 survey suggested this species was likely more abundant on Phillip Island than in 1978, with a population estimate of between 99,000 and 176,000 (Cogger et al. 2006). The growth in range and abundance was considered due to revegetation and expanded habitat.
The distribution within the Norfolk Island Group is shown in Map 16.
Map 16 Distribution of Christinus guentheri
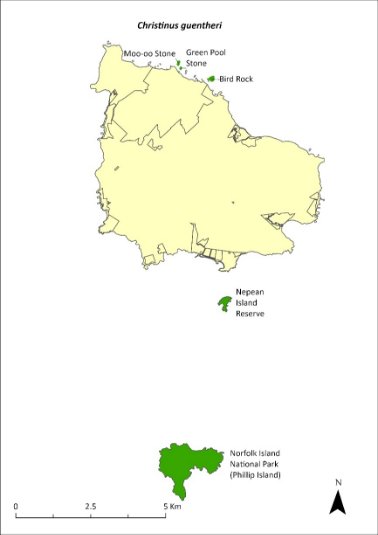
Green shading indicates the islands on which the species has been recorded (Cogger et al. 2006).
Ecology
A nocturnal species that shelters under rocks, in splits in trees, and under man-made shelter during the day. It feeds on beetles, spiders, moths, ants and other insects among the leaf litter; it also hunts in trees and feeds on the nectar of some flowers. It uses rock boulders and rock crevices for shelter and egg-deposition sites. Lays a clutch of 1–3 eggs, and incubation is about 80 to 90 days. Females probably have multiple clutches as gravid females have been reported in November and in March. It forms a significant portion of the prey for the Phillip Island centipede (Cormocephalus coynei; Halpin et al. 2021b), but predation is unlikely to be impacting overall population levels.
Habitat
The species occurs in a range of habitats including primary forest, secondary regrowth forest and lightly grassed or bare rocky islands that are exposed to extreme climatic and physical conditions (Cogger et al. 2006). It has been observed at night on both shrubs and trees but especially on flowering white oak (Lagunaria patersonia) and Phillip Island hibiscus (Hibiscus insularis), where it feeds on the nectar (Cogger et al. 2006). It can also be found on Norfolk Island pine (Araucaria heterophylla) and on the weed species African olive (Olea europaea cuspidata), but it is largely absent from all but the edges of the dense groves of immature olives. Most geckos make only relatively short journeys onto bare ground from the cover of edge vegetation or rock screes.
Threats
The presence of rats and cats on Norfolk Island probably prevents this species from establishing there. The main threats are the introduction of predators (such as rats and cats) or competitors such as the Asian house gecko (Hemidactylus frenatus) to Phillip and Nepean Islands, and degradation and loss of habitat on those islands.
Impact on other species
None known.
Risk assessment
The risk assessment is shown in Table 43.
Table 43 Risk assessment for Christinus guentheri
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Minor | Medium |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Negligible | Negligible |
4. Degradation of native vegetation through current or future grazing | Rare (0–10%) | Negligible | Negligible |
6. Predation by rodents | Rare (0–10%) | Negligible | Negligible |
7. Predation by cats | Rare (0–10%) | Minor | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Possible (26–50%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Major | Low |
11. Competition from/change of habitat because of weed invasion | Almost certain (91–100%) | Negligible | Negligible |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens a | Rare (0–10%) | Extreme | Medium |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Almost certain (91–100%) | Minor | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Rare (0–10%) | Minor | Negligible |
a Assessment is of risk of potential new species reaching Phillip Island.
Management actions
Continue weed control and habitat restoration work on Phillip Island, particularly removal of African olive and re-establishing stands of white oak; and establish effective quarantine protocols for Phillip Island. If rats and cats can be controlled on Norfolk Island, it may be possible to re-establish a population there. Develop captive breeding protocols for the species so that the necessary procedures are in place if a translocation to another island is required in the future. Identify other islands where the Norfolk Island reptiles could be translocated to provide an insurance population.
The Lord Howe Island Biodiversity Management Plan covers the recovery needs of this species across its range outside of Norfolk Island. Possible future actions (such as captive breeding and translocation) may need to be undertaken in collaboration with the NSW Government as appropriate.
Recovery target
The recovery target is shown in Table 44.
Table 44 Recovery target for Christinus guentheri
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Vulnerable | 176,000 | 93% in the national park 5% in public reserves 2% in other lands | Maintained numbers and range |
Relevant literature
Chapple D, Tingley R, Mitchell N, Macdonald S, Keogh JS, Shea G, Bowles P, Cox N & Woinarski J (2019) The Action Plan for Australian Lizards and Snakes 2017. CSIRO Publishing, Melbourne.
Cogger HG, Cameron EE & Sadlier RA (1979) The terrestrial reptiles of islands in the Norfolk Island complex. Unpublished report to the Australian National Parks and Wildlife Service, Canberra.
Cogger HG, Muir G & Shea G (2006) A survey of the terrestrial reptiles of Norfolk Island March 2005: Report 4. Assessment of the suitability of potential gecko re-introduction sites on Norfolk’s main island and a review of threatening processes and recovery actions proposed in the draft Recovery Plan. Unpublished report to the Department of the Environment and Heritage, Canberra.
Halpin LR, Terrington DI, Jones HP, Mott R, Wong WW, Dow DC, Carlile N & Clarke RH (2021) Arthropod predation of vertebrates structures trophic dynamics in island ecosystems. The American Naturalist 198(4), 540–550.
Oligosoma lichenigerum—Lord Howe Island skink
Conservation significance
Endemic to the Norfolk Island Group and the Lord Howe Island Group.
EPBC Act Listing Status: Vulnerable
State Listing Status: Listed as Vulnerable under the Biodiversity Conservation Act 2016 (NSW).
Non-statutory Listing Status: Listed as Vulnerable under The Action Plan for Australian Lizards and Snakes 2017 (Chapple et al. 2019)
For further information on the species outside of the Norfolk Island Group, see the species profile on SPRAT.
Distribution
The Lord Howe Island skink (Oligosoma lichenigerum) was described in 1874 from Lord Howe Island and was first recorded on the Norfolk Island complex in 1978 (Cogger et al. 1993). On the Lord Howe Island Group this species is as widely distributed as the Lord Howe Island gecko (Christinus guentheri).
On the Norfolk Island Group this species has only been found on Phillip Island, despite considerable search effort on Nepean Island and on many of the small rocky islets (Cogger et al. 1979). The species is not as abundant as the Lord Howe Island gecko; for example, 10 specimens were encountered on Fisherman’s Hut Rock on Phillip Island in 1979, during which time 285 geckos were also found (Cogger et al. 1979).
The population on Phillip Island is estimated to be large and secure, and the increase in suitable habitat since the removal of rabbits in 1986 suggests that they were at least as abundant in 2005 as they were in 1978 (Cogger et al. 2006). The distribution of the species is shown in Map 17.
Ecology
Knowledge of the biology, ecology and conservation status of this species is fragmentary and based on few individual records. It is a nocturnal species that shelters under rocks, in splits in trees, and in holes in rocks during the day. It feeds on beetles, spiders, moths, ants and other insects among the leaf litter.
Habitat
This species ranges across a variety of habitats from bare cliffs and eroded slopes to the narrow and heavily wooded gullies of Long Valley (Cogger et al. 1993). Greater densities of skinks occur where the vegetation has formed dense root mats in which they could hide and forage, sometimes of grasses but especially of Moo-oo (Cyperus lucidus) and native flax (Phormium tenax).
Map 17 Distribution of Oligosoma lichenigerum
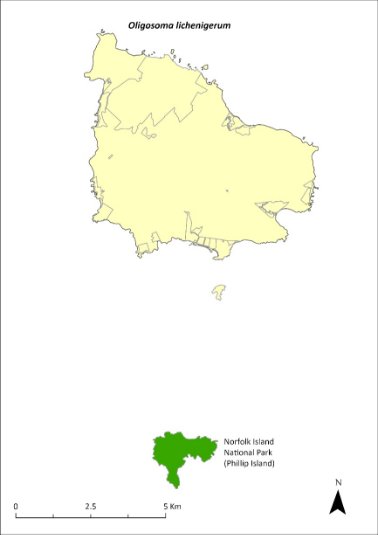
Green shading indicates the islands on which the species has been recorded (Cogger et al. 2006).
Threats
The presence of rats and cats on Norfolk Island probably prevents this species from establishing there. The main threats are the introduction of predators (such as rats and cats) or potential competitors to Phillip Island, and degradation and loss of habitat on the island. Drying conditions and lower soil moisture balances due to climate change are also a threat.
Impact on other species
None known.
Risk assessment
The risk assessment is shown in Table 45.
Table 45 Risk assessment for Oligosoma lichenigerum
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Moderate | High |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Moderate | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Moderate | High |
4. Degradation of native vegetation through current or future grazing | Rare (0–10%) | Moderate | Negligible |
6. Predation by rodents | Rare (0–10%) | Negligible | Negligible |
7. Predation by cats | Rare (0–10%) | Minor | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Possible (26–50%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Major | Low |
11. Competition from/change of habitat because of weed invasion | Almost certain (91–100%) | Negligible | Negligible |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens a | Rare (0–10%) | Extreme | Medium |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Almost certain (91–100%) | Major | Extreme |
15. Problems caused by small populations, including lack of genetic diversity | Possible (26–50%) | Moderate | Medium |
a Assessment is of risk of potential new species reaching Phillip Island.
Management actions
Continue weed control and habitat restoration work on Phillip Island, and establish effective quarantine protocols. If rats and cats can be controlled on Norfolk Island, it may be possible to establish a population there. Develop captive breeding protocols for the species so that the necessary procedures are in place if a translocation to another island is required in the future. Identify other islands where the Norfolk Island reptiles could be translocated to provide an insurance population.
The Lord Howe Island Biodiversity Management Plan covers the recovery needs of this species across its range outside of Norfolk Island. Possible future actions (such as captive breeding and translocation) may need to be undertaken in collaboration with the NSW Government as appropriate.
Recovery target
The recovery target is shown in Table 46.
Table 46 Recovery target for Oligosoma lichenigerum
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Vulnerable | 7,000 | 100% within the national park | Increase in area of occupancy by at least 10% |
Relevant literature
Chapple D, Tingley R, Mitchell N, Macdonald S, Keogh JS, Shea G, Bowles P, Cox N & Woinarski J (2019) The Action Plan for Australian Lizards and Snakes 2017. CSIRO Publishing, Melbourne.
Cogger HG, Cameron EE & Sadlier RA (1979) The terrestrial reptiles of islands in the Norfolk Island complex. Unpublished report to the Australian National Parks and Wildlife Service, Canberra.
Cogger HG, Cameron EE, Sadlier RA & Eggler P (1993) The Action Plan for Australian Reptiles. Australian Nature Conservation Agency, Canberra.
Cogger HG, Muir G & Shea G (2006) A survey of the terrestrial reptiles of Norfolk Island March 2005: Report 4. Assessment of the suitability of potential gecko re-introduction sites on Norfolk’s main island and a review of threatening processes and recovery actions proposed in the draft Recovery Plan. Unpublished report to the Department of the Environment and Heritage, Canberra.
Cyanoramphus cookii—Norfolk Island green parrot
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Endangered.
Non-statutory Listing Status: Described as Critically Endangered in the Action Plan for Australian Birds 2020 (Garnett & Baker 2021).
Approved Conservation Advice: 15/07/2016 (TSSC 2016a).
Distribution and abundance
The Norfolk Island green parrot was a common forest bird when Norfolk Island was discovered in the late 1700s; however, by the late 1970s, fewer than 50 individuals remained and the population was restricted to the national park (Schodde et al. 1983; Hermes et al. 1986; Hill 2002).
In 1983, the Government Conservator commenced a captive breeding program, which was followed by sustained rat and cat control as well as artificial nest construction (Hicks & Greenwood 1989). Over 15 years, between 1987 and 2002, approximately 250 chicks fledged successfully, and sightings became common. However, following a period of no active management between 2007 and 2013, a survey indicated the population had declined to between 42 and 96 individuals, of which only 10 were confirmed adult females (Ortiz-Catedral 2013). As a result of renewed efforts to provide rat‑ and cat-proof nests and intensify control of rats and cats, nest success increased substantially (Ortiz-Catedral et al. 2018) and the population increased to an estimated 438 (SE ± 168) in 2017 (Skirrow 2019). However, it should be noted that there are large confidence intervals around this estimate, and while there certainly appears to have been population growth, the rate of increase and current population size are not clear (Macgregor et al. 2021).
The Mt Pitt section of Norfolk Island National Park remains a stronghold for the species, but there is growing anecdotal evidence that its range has increased substantially, and Norfolk Island green parrots are now regularly seen in areas well outside of the national park boundary.
The distribution is shown in Map 18.
Map 18 Distribution of Cyanoramphus cookii
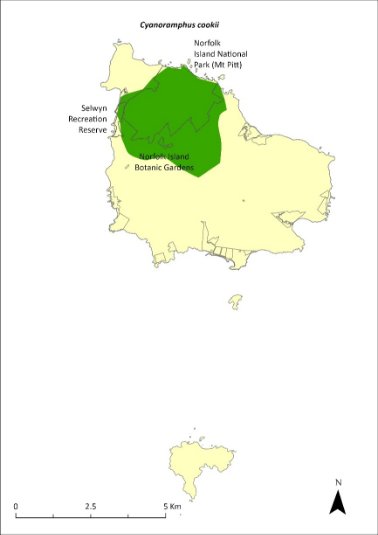
The stronghold for the Norfolk Island green parrot is within the shaded area; however, its range extends across Norfolk Island (Director of National Parks 2010, NIRC 2020).
Ecology
Breeds in all months of the year. Hicks and Greenwood (1990) reported a peak between December to March; more recent data from 2013–20 indicate a peak from January to June (Director of National Parks unpublished). Average clutch size is six eggs (1–8; Hicks & Greenwood 1989; Director of National Parks unpublished), and individual pairs can successfully fledge young up to four times in a single year (Hill 2002). Similar to other Cyanoramphus species, females incubate the eggs and undertake most of the chick feeding, while males provide food for nesting females (Greene 2003; Ortiz-Catedral et al. 2009).
Nests in hollows of living trees often within two metres of the ground or at ground level among tree roots. Adults return to the same nest site each season but will also use other sites within their territory.
Diet is a variety of seeds, fruits, flowers, pollen, sori, sprout rhizomes and bark, taken from at least 30 native and introduced plant species.
Active foraging is mostly at heights of 2–7 metres, although the parrots also feed on the ground, especially in winter (Waldman 2016). They have some overlapping dietary preferences with the crimson rosella, although there are seasonal differences (Simmonds 2019).
Habitat
The breeding range is thought to be largely restricted to the Mt Pitt section of the national park, though successful nesting has been recorded outside the park (D Gautschi 2024. pers comm 12 January). The species forages in the park and adjacent forested areas and orchards.
Threats
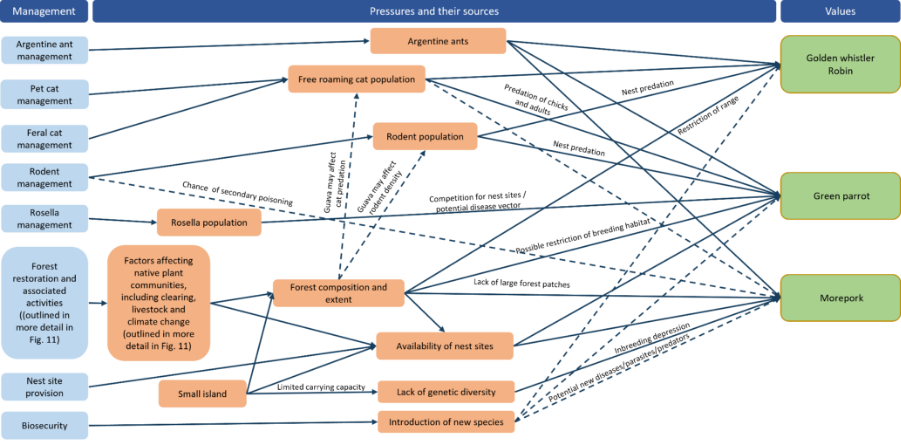
The main factors responsible for the decline of the species were clearance of vegetation for agriculture, particularly trees with suitable nesting hollows, and predation from introduced predators. Change in forest structure due to weed invasion is likely to also have reduced the area of suitable habitat available to the species (Garnett et al. 2011; TSSC 2016a). Predation of eggs and chicks by rats and cats, a shortage of suitable predator-free nest sites, and nest hollow competition from introduced crimson rosellas (which have a population three times that of the Norfolk Island green parrot (TSSC 2016a; Skirrow 2019)) are the main factors limiting population recovery (Macgregor et al. 2021), while disease may be a significant cause of mortality in certain circumstances (Hill 2002). The purple swamphen, a self-introduced species that arrived on the Norfolk Island Group before 1888, may prevent re-establishment on Phillip Island (Heinsohn 2019).
Impact on other species
None known.
Risk assessment
The risk assessment is shown in Table 47.
Table 47 Risk assessment for Cyanoramphus cookii
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Moderate | High |
2. Loss and fragmentation of native vegetation through current or future land clearing | Unlikely (11–25%) | Minor | Low |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Moderate | High |
4. Degradation of native vegetation through current or future grazing | Likely (51–90%) | Minor | Medium |
5. Lack of available nest sites | Almost certain (91–100%) | Moderate | High |
6. Predation by rodents | Almost certain (91–100%) | Major | Extreme |
7. Predation by cats | Almost certain (91–100%) | Major | Extreme |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Moderate | Negligible |
10. Predation by Argentine ant | Likely (51–90%) | Moderate | Medium |
11. Competition from/change of habitat because of weed invasion | Possible (26–50%) | Negligible | Negligible |
12. Infection by pathogens already present | Likely (51–90%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Possible (26–50%) | Moderate | Medium |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Likely (51–90%) | Major | High |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Moderate | High |
Management actions
Control predators and competitors, particularly feral cats, black rats and rosellas (TSSC 2016). Implement targeted control of cats and rats to reduce predation on the Norfolk Island green parrot. Provide and maintain suitable rat proof nest sites, and optimise the number, placement and spatial configuration of these nest sites, particularly outside the national park (TSSC 2016). Continue weed control and forest rehabilitation work (particularly Nestegis dominant forest) and protect old hollow-bearing trees (TSSC 2016). Control purple swamphens on Phillip Island to facilitate re-establishment of Norfolk Island green parrots. Develop approaches to help the Norfolk Island community manage the species’ impacts on orchards as the population continues to expand beyond the park. Explore options for translocation of the species to other islands to create insurance populations.
Recovery target
The recovery target is shown in Table 48.
Table 48 Recovery target for Cyanoramphus cookii
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Endangered | 438 (270–606) | 52% in the national park 1% in public reserves 47% in other lands | The population has increased to 1000 individuals, including 150 to 200 breeding pairs, and the breeding range has extended to the south of the island |
Relevant literature
Garnett ST & Baker GB (2021) The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne.
Garnett ST, Szabo J & Dutson G (2011) The Action Plan for Australian Birds 2010. CSIRO Publishing, Melbourne.
Gautschi D (2024) personal communication by email, 12 January. Australian National University.
Greene TC (2003) Breeding biology of red-crowned parakeets (Cyanoramphus novaezelandiae novaezelandiae) on Little Barrier Island, Hauraki Gulf, New Zealand. Notornis 50, 83–99.
Heinsohn R (2019) Review of the translocation of Norfolk Island Green Parrots from Norfolk Island to Phillip Island. Report to the Director of National Parks, Canberra.
Hermes N, Evans O & Evans B (1986) Norfolk Island birds: a review 1985. Notornis 33, 141–149.
Hicks J & Greenwood D (1990) Rescuing Norfolk Island’s Parrot. Birds International 2, 35–47.
Hicks J & Preece M (1991) Green Parrot. 1991 Recovery Plan. Unpublished report to the Australian National Parks and Wildlife Service.
Hill R (2002) Recovery Plan for the Norfolk Island Green Parrot Cyanoramphus novaeseelandiae cookii. Environment Australia, Canberra.
Macgregor NA, Wilson M, Brown SM, Goumas M, Heinsohn R, Clarke RH, Ortiz-Catedral L, Greenup N, Christian M, Greenwood D, Ward R & Garnett ST (2021) Norfolk Island Green Parrot Cyanoramphus novaezelandiae cookii, in ST Garnett & GB Baker (eds), The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne. pp. 432-435.
Ortiz-Catedral L (2013) The Population and Status of Green Parrot (Tasman Parakeet) Cyanoramphus cookii on Norfolk Island. Unpublished report to the Director of National Parks.
Ortiz-Catedral L, Kearvell JC, Hauber ME & Brunton DH (2009) Breeding biology of the critically endangered Malherbe’s parakeet on Maud Island, New Zealand, following the release of captive-bred individuals. Australian Journal of Zoology 57, 433–439.
Ortiz-Catedral L, Nias R, Fitzsimons J, Vine S & Christian M (2018) Back from the brink–again: the decline and recovery of the Norfolk Island green parrot, in S Garnett, P Latch, D Lindenmayer & J Woinarski (eds), Recovering Australian Threatened Species: A Book of Hope. CSIRO Publishing.
Schodde R, Fullagar P & Hermes N (1983) A review of Norfolk Island birds past and present (Special Publication No. 8). Australian National Parks and Wildlife Service, Canberra.
Simmonds SA (2019) Habitat use by Tasman Parakeets (Cyanoramphus cookii) and Crimson Rosellas (Platycercus elegans) on Norfolk Island, South Pacific. MSc Thesis, Massey University, Auckland.
Skirrow MJ (2019) Estimating the population size of two critically endangered South Pacific parakeets: the Tasman Parakeet and Malherbe's Parakeet. MSc Thesis, Massey University, Auckland.
Smithers CN & Disney HJ (1969) The distribution of terrestrial and freshwater birds on Norfolk Island. Australian Zoologist 15, 127–140.
Threatened Species Scientific Committee (TSCC) (2016a) Conservation Advice Cyanoramphus cookii Norfolk Island green parrot. Department of the Environment, Canberra.
Waldmann A (2016) Foraging ecology of the world's only population of the critically endangered Tasman parakeet (Cyanoramphus cookii) on Norfolk Island. MSc Thesis, Massey University, Auckland.
Ninox novaeseelandiae undulata—Norfolk Island morepork
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Endangered.
Non-statutory Listing Status: Described as Critically Endangered in the Action Plan for Australian Birds 2020 (Garnett & Baker 2021).
Approved Conservation Advice: 15/07/2016 (TSSC 2016b).
Distribution and abundance
The Norfolk Island morepork (or boobook owl) was first recorded by King in 1788–90. Since 1909 the owl had been recorded as occurring largely in the gullies surrounding Mt Pitt (Smithers & Disney 1969; Olsen et al. 1989). A reasonable population remained in 1912–13 but by 1968 the owl was considered extremely rare and was heard only occasionally (Turner et al. 1968; Smithers & Disney 1969). By 1986 the population had declined to a single female, and the genetically pure form of the species is now extinct (TSSC 2016b).
Two males from the closely related New Zealand subspecies were introduced in 1987. In 1989 the Norfolk female and one of the New Zealand males raised their first chicks. They also produced chicks in 1990 but those were the last chicks produced by the Norfolk female, and she was last recorded in October 1995. There has been subsequent second and third generation breeding with 45 ‘hybrid’ offspring banded up to December 2007. The current population is entirely descended from that single breeding pair: the last female Norfolk Island morepork Ninox n. undulata and one of the introduced males N. n. novaeseelandiae (Olsen et al. 1989).
Successful breeding was observed in every year from 1993 to 2007. Subsequently, a single successful breeding event was observed between 2008 and 2018 (successful breeding in 2011 only). In 2016, there were estimated to be 32 individuals (Wilson 2016); estimates from more recent surveys reported a population of 20–30 (Sperring et al. 2021a). After the establishment of new nest boxes, one nest found in 2019 produced two fledglings, while a single nest found in 2020 (believed to be from the same pair and in a box near the location of the successful nest in 2019) had eggs that did not hatch (Sperring et al. 2021b). Although surveys in 2019–2021 detected just two previously banded birds of the 12 captured, indicating that undetected breeding has occurred at some point, the population possibly consists of ageing birds that are not reproducing at a sufficient rate to maintain the population. In December 2023, a nest with two new chicks was discovered and was being monitored.
The population is now fairly evenly distributed across the entire national park with a higher density on the southern slopes of Mt Pitt and Mt Bates. Tracking data from spring 2019 and 2020 showed that the average territory size for owls living mostly within the national park was 48 hectares while the average size for owls outside of the park was 128 hectares. Territory sizes during winter are similar to those in spring, though one owl tracked during winter, and displaying behaviour suggestive of searching for a mate, ranged over an area of 389 hectares. Because owls occupy small territories in the national park, the population density is much higher there; owls are distributed more sparsely across the rest of the island (Sperring et al. 2021b).
Whilst Norfolk Island moreporks have previously been heard on Phillip Island, they are not currently known to occupy the island (M Wilson 2024. pers comm 12 January). All recent breeding has taken place in Norfolk Island National Park (Sperring 2021a,b).
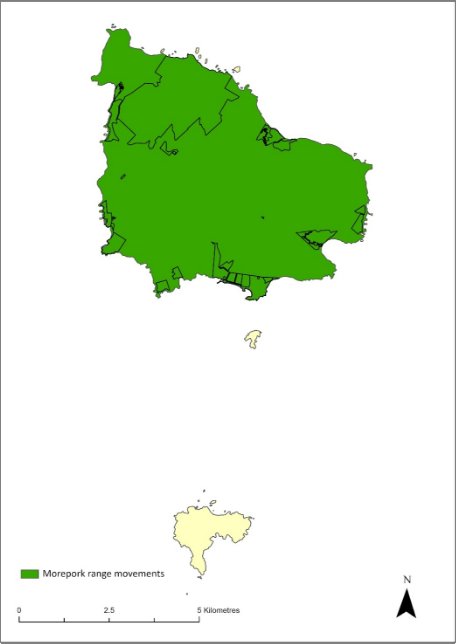
The distribution is shown in Map 19.
Map 19 Range movements of the Norfolk Island morepork on Norfolk Island
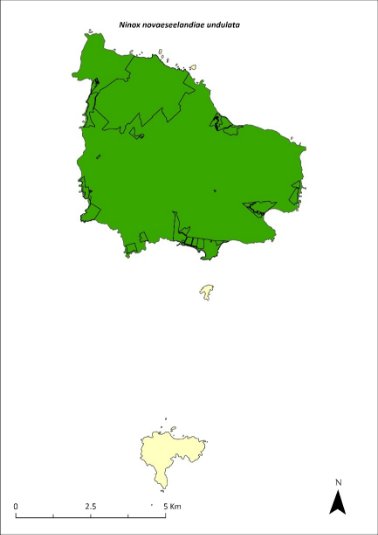
Source: Sperring et al. 2021.
Ecology
Breeds September to January. Clutch size can be up to three eggs, but two eggs per clutch is more common.
Nests in tree hollows. All nests of the hybrid population have been in artificial nest boxes, although breeding is suspected to have occurred in natural hollows.
Feed primarily on insects, in particular orthopterans and coleopterans, as well as rodents, passerines (including the Norfolk Island robin and slender billed white-eye) and white terns (Olsen 1996; Sperring et al. 2001b).
Habitat
Norfolk Island moreporks prefer native woody vegetation, red guava (Psidium cattleyanum) or Eucalyptus plantation to open land and other woody weeds. They also prefer canopy height above 10 m (Sperring, unpublished data), mostly roosting high in the canopy. They are most commonly found in native trees (particularly ironwood and bloodwood) but have also been seen roosting in guava, olive and banana plantations.
Threats
The decline of the Norfolk Island morepork was probably caused by a combination of unrelated environmental, demographic and genetic forces acting on a naturally small population. The main factors were likely to have been: the loss of approximately 30 individuals from the population for a natural history collection in 1913; the loss of suitable habitat and nesting hollows caused by land clearing and selective logging of large trees; and competition for hollows from introduced species such as crimson rosellas and European starlings (TSSC 2016b). Current major threats include inbreeding depression, lack of suitable nesting sites, and competition from introduced species (TSSC 2016b). Low habitat suitability across the island is also likely to reduce the carrying capacity of the island putting pressure on the population to maintain genetic diversity (Sperring et al. 2021a). Secondary poisoning from rodent and chicken baiting is also a threat (likely cause of death of two chicks in 2012 (Debus 2012) and near death of one likely poisoned adult in 2021 (Sperring et al. 2021b). Predation of eggs and chicks by rats, cats and Argentine ants is also a possible threat. Weed invasion by red guava (Psidium cattleyanum), African olive (Olea europaea), wild tobacco (Solanum mauritianum) and lantana (Lantana camara) and the resulting change in forest structure is also likely to affect owls’ ability to hunt (Wilson 2016).
Impact on other species
None known.
Risk assessment
The risk assessment is shown in Table 49.
Table 49 Risk assessment for Ninox novaeseelandiae undulata
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Major | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Almost certain (91–100%) | Major | Extreme |
3. Degradation of native vegetation through past grazing or loss of nutrients | Likely (51–90%) | Major | High |
4. Degradation of native vegetation through current or future grazing | Likely (51–90%) | Major | High |
5. Lack of available nest sites | Possible (26–50%) | Major | High |
6. Predation by rodents | Unlikely (11–25%) | Minor | Low |
7. Predation by cats | Unlikely (11–25%) | Minor | Low |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Likely (51–90%) | Minor | Medium |
11. Competition from/change of habitat because of weed invasion | Possible (26–50%) | Moderate | Medium |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Rare (0–10%) | Minor | Negligible |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Likely (51–90%) | Minor | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Extreme | Extreme |
16. Secondary poisoning | Likely (51–90%) | Moderate | Medium |
Management actions
Maintain suitable nest boxes (particularly in appropriate locations) to improve the breeding success of individuals within the population (TSSC 2016b). Restore habitat outside of the national park to increase the carrying capacity of the island and reduce the pressure of maintaining genetic diversity (TSSC 2016b). Protect old hollow bearing trees. Maintain crimson rosella control program to minimise competition for nest boxes (TSSC 2016b). Genetic rescue through the introduction of individuals from New Zealand or Australia may be required in future. Prevention or serious reduction in the use of second-generation rodent and chicken baits outside of the national park is also likely to assist the population.
Recovery target
The recovery target is shown in Table 50.
Table 50 Recovery target for Ninox novaeseelandiae undulata
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Endangered | 25 (20–40) | 9% in the national park 1% in public reserves 86% in other land | The population size has increased by at least 30% from 2023, breeding is occurring both inside and outside of the national park. |
Relevant literature
Debus SJS (2012) Norfolk Island Boobook chick deaths. Boobook 30, 6.
Garnett ST & Baker GB (2021) The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne.
Olsen P (1996) Re-establishment of an endangered subspecies: the Norfolk Island Boobook. Bird Conservation International 6, 63–70.
Olsen P (1997) Recovery Plan for the Norfolk Island Boobook Owl Ninox novaeseelandiae undulata. Environment Australia, Canberra.
Olsen PD (1986) Status and conservation of the Norfolk Island Boobook Owl Ninox novaeseelandiae undulata. Unpublished report to the Australian National Parks and Wildlife Service.
Olsen PD, Mooney NJ & Olsen J (1989) Status and conservation of the Norfolk Island Boobook Ninox novaeseelandiae undulata, in BU Meyburg & RD Chancellor (eds), Raptors in the Modern World. WWGBP, Berlin. pp. 123–129.
Schodde R, Fullagar P & Hermes N (1983) A review of Norfolk Island birds past and present (Special Publication No. 8). Australian National Parks and Wildlife Service, Canberra.
Smithers CN & Disney HJ (1969) The distribution of terrestrial and freshwater birds on Norfolk Island. Australian Zoologist 15, 127–140.
Sperring F, Webster W, Isaac B, Clarke R, Gautschi D, Heinsohn R, Olsen P, Weeks A, Macgregor N, Wilson M & Greenup N (2021b) Ecology, genetics, and conservation management of the Norfolk Island morepork and green parrot. Interim report to the NESP Threatened Species Recovery Hub, Brisbane.
Sperring VF, Brown SM, Macgregor NA, Olsen P, Clarke RH, Wilson M, Greenup N, Weeks A, Ward R, Greenwood D, Christian M & Garnett ST (2021a) Norfolk Island Morepork Ninox novaeseelandiae undulata, in ST Garnett & GB Baker (eds), The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne. pp. 360-363.
TSSC (Threatened Species Scientific Committee) (2016b). Conservation Advice Ninox novaeseelandiae undulata Norfolk Island boobook owl. Department of the Environment, Canberra.
Turner JS, Smithers CN & Hoogland RD (1968) The Conservation of Norfolk Island. Australian Conservation Foundation, Melbourne.
Wilson M (2016) Owl Survey Report, December 2016. Director of National Parks, Canberra.
Wilson M (2024) personal communication by email, 12 January, Parks Australia (Norfolk Island National Park).
Pachycephala pectoralis xanthroprocta—Norfolk Island golden whistler (tamey)
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Vulnerable.
Non-statutory Listing Status: Described as least concern in the Action Plan for Australian Birds 2020 (Garnett & Baker 2021).
Distribution and abundance
This subspecies was originally distributed throughout Norfolk Island (and probably Phillip Island) but experienced range contraction from the 1960s (Schodde et al. 1983) and became largely restricted to the Mt Pitt section of the national park and nearby forested areas by the late 1980s (Bell 1990).
There were about 1,000 breeding birds in 1987, with some evidence of a decrease in numbers outside the park between 1987 and 1996 (Robinson 1988, 1997).
Dutson (2013) estimated the population size at 1400–3650 mature individuals in 2009. The most recent estimate is 1372–1970 individuals in 2019 (Nance et al. 2021a). The population is thought to have been broadly stable since 2009; however, the confidence in that trend is low. There have been records from many sites outside the boundaries of the park over the last decade (Nance et al. 2021a).
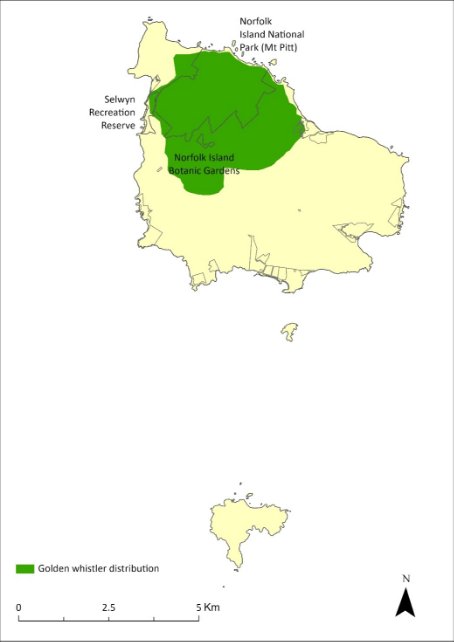
The distribution is shown in Map 20.
Ecology
Breeds September to November, nest with one egg in December, young present in February. Nests in small trees or in hanging masses of vines.
Diet poorly known but consists of insects and some fruit. Often ventures onto the ground to forage in leaf litter.
Habitat
The Norfolk Island golden whistlers occur in all vegetation types within the national park, including rainforest, palm forest and pine forest, but are most abundant in palm forest (Robinson 1988, 1997; Major 1989). Outside the park, they sometimes occur in remnant forest on agricultural land. The subspecies generally inhabits the shrubby understorey (Robinson 1988).
Map 20 Distribution of Pachycephala pectoralis xanthroprocta
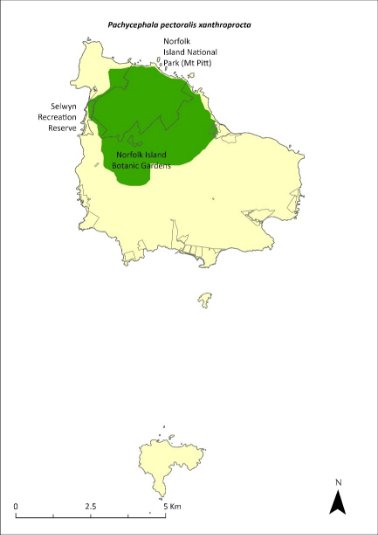
The shaded area indicates the approximate current range of the golden whistler (Director of National Parks 2010, NIRC 2020).
Threats
The main threats to the remaining population of Norfolk Island golden whistlers is predation from black rats and Argentine ants, with predation by cats a likely additional pressure. Whistlers may be more vulnerable to rat predation in disturbed environments (Nance et al. 2021a). The limited extent of native vegetation outside protected areas represents a barrier to the species recolonising its former range.
Impact on other species
None known.
Risk assessment
The risk assessment is shown in Table 51.
Table 51 Risk assessment for Pachycephala pectoralis xanthroprocta
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Moderate | High |
2. Loss and fragmentation of native vegetation through current or future land clearing | Unlikely (11–25%) | Moderate | Low |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Minor | Medium |
4. Degradation of native vegetation through current or future grazing | Almost certain (91–100%) | Minor | Medium |
5. Lack of available nest sites | Rare (0–10%) | Minor | Negligible |
6. Predation by rodents | Almost certain (91–100%) | Moderate | High |
7. Predation by cats | Likely (51–90%) | Moderate | Medium |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Almost certain (91–100%) | Moderate | High |
11. Competition from/change of habitat because of weed invasion | Almost certain (91–100%) | Minor | Medium |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Minor | Low |
15. Problems caused by small populations, including lack of genetic diversity | Unlikely (11–25%) | Minor | Low |
Management actions
Control the main predators, black rats and feral cats, with targeted control of rodents in natural areas to protect Norfolk Island golden whistler nests (predation by rats has a strong influence on fledging rates). Restore native forest inside the national park, but also outside the park, with patches of appropriate size, composition, and physical connectedness, to enable passerine birds to expand their range.
Recovery target
The recovery target is shown in Table 52.
Table 52 Recovery target for Pachycephala pectoralis xanthroprocta
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Vulnerable | 1,671 (1,372–1,970) | 42% in the national park 1% in public reserves 57% in other lands | The population is at least 2000 individuals and distributed across Norfolk Island |
Relevant literature
Bell BD (1990) The status and management of the White-breasted White-eye and other birds of Norfolk Island. Unpublished report to the Australian Nature Conservation Agency.
Commonwealth of Australia (2005) National Recovery Plan for the Norfolk Island Scarlet Robin Petroica multicolor multicolor and the Norfolk Island Golden Whistler Pachycephala pectoralis xanthroprocta. Department of the Environment and Heritage.
Dutson G (2013) Population densities and conservation status of Norfolk Island forest birds. Bird Conservation International 23, 271–282.
Garnett ST & Baker GB (2021) The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne.
Garnett ST & Crowley GM (2000) The Action Plan for Australian Birds. Environment Australia.
Major R (1989) Reproductive output and recruitment of the Norfolk Island Scarlet Robin (Petroica multicolor multicolor) Phase II. Report to the Australian National Parks and Wildlife Service, Canberra.
Nance AH, Mitchell W, Wilson M, Brown SM, Clarke RH, Macgregor NA, Ward R & Garnett ST (2021a) Norfolk Island Golden Whistler Pachycephala pectoralis xanthoprocta, in ST Garnett & GB Baker (eds), The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne. pp. 709-710.
Robinson D (1988) Ecology and Management of the Scarlet Robin, White-breasted White-eye, and Long-billed White-eye of Norfolk Island. Consultants’ report to the Australian National Parks and Wildlife Service, Canberra.
Robinson D (1997) An evaluation of the status of the Norfolk Island Robin following rat-control and weed-control works in the Norfolk Island National Park. Report to Environment Australia, Canberra.
Schodde R, Fullagar P & Hermes N (1983) A review of Norfolk Island birds past and present (Special Publication No. 8). Australian National Parks and Wildlife Service, Canberra.
Petroica multicolor—Norfolk Island robin
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Vulnerable.
Non-statutory Listing Status: Described as Endangered in the Action Plan for Australian Birds 2020 (Garnett & Baker 2021).
Distribution and abundance
The endemic Norfolk Island robin was once common and widespread over Norfolk Island (and probably also on Phillip Island), but its range has contracted significantly since about 1960 (Schodde et al. 1983; Robinson 1988). The remaining population is almost entirely confined to Norfolk Island National Park and a few adjacent forested areas including some private properties and Selwyn Reserve (Robinson 1988, 1997; Major 1989; Bell 1990; M Christian 2024. pers comm 12 January).
There was little change in the density of birds inside the national park from 1987 to 1996, and the population appeared likely to remain stable if predator control continued (Robinson 1997).
There were estimated to be 750 mature Norfolk Island robins in the park in 2018 (about 375 pairs; Dawlings & Clarke unpublished report cited in Nance et al. 2021b) compared with 520 pairs (1040 individuals) in 1987 and 380–440 pairs (760–880 individuals) in 1996 (Robinson 1988).
Other important sites outside the park include the valleys between Prince Phillip Drive and Mt Pitt Road, between Douglas Drive and the park boundary, and valleys near Duncombe Bay (Commonwealth of Australia 2005). Occasional sightings elsewhere on the island since 2017 (for example a private rehabilitation site above Bloody Bridge) probably represent dispersing individuals (Nance et al. 2021b).
The distribution is shown in Map 21.
Ecology
Breeding season is late September to March. The species can breed in the first year of life and produce two eggs per season. On average each pair produces one fledgling per year (Major 1989). Nests placed near the top of the subcanopy or in upright fork or horizontal branch of tree.
Feeds on invertebrates, mainly insects, foraging on the ground in deep litter where a dense understorey has an open ground layer (Robinson 1988) or using low horizontal branches from which to pounce on prey.
Map 21 Distribution of Petroica multicolor
The shaded area indicates the approximate current range of the Norfolk Island robin (Director of National Parks 2010, NIRC 2020.
Habitat
Mainly inhabits the cooler and damper native rainforest with lower densities in habitats dominated by Norfolk Island palm (Rhopalostylis baueri), African olive (Olea europaea cuspidata) or eucalypt plantations (Robinson 1988, 1997; Major 1989). The species generally prefers areas such as gullies with a deep moist litter layer, dense shrub layer 1–10 m tall to provide shelter and nests, and an open shaded layer near ground level to provide visibility for foraging (Robinson 1988, 1997).
Threats
The primary threat to survival of the population is predation from black rats. In a study in which nests were observed with remote cameras, rats preyed on 75% of nests and reduced nest success to 17%. In unbaited areas, rodent density was twice as high (8.1/ha cf 4.2/ha) and robin nest survival approximately 20 times lower (1.6% cf. 36.4%) than in baited areas (Dawlings and Clarke unpublished reported in Nance et al. 2021b). Population viability modelling suggests decline to extinction within six years in the absence of rat baiting (based on observed nest survival rates in unbaited areas; Nance et al. 2021b). Predation by cats and Argentine ants are also a threat. The invasive red guava (Psidium cattleyanum) is likely to increase rat populations by providing unlimited food supply for rats 3–4 months of the year (Nance et al. 2021b). A possible trend towards drier conditions in a changing climate (Bureau of Meteorology 2019) could affect the forest habitat of the Norfolk Island robin and the abundance of invertebrates they rely on for food (Nance et al. 2021b)
Impact on other species
None known.
Risk assessment
The risk assessment is shown in Table 53.
Table 53 Risk assessment for Petroica multicolor
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Major | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Unlikely (11–25%) | Moderate | Low |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Negligible | Negligible |
4. Degradation of native vegetation through current or future grazing | Almost certain (91–100%) | Negligible | Negligible |
5. Lack of available nest sites | Rare (0–10%) | Minor | Negligible |
6. Predation by rodents | Almost certain (91–100%) | Major | Extreme |
7. Predation by cats | Likely (51–90%) | Major | High |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Almost certain (91–100%) | Moderate | High |
11. Competition from/change of habitat because of weed invasion | Almost certain (91–100%) | Minor | Medium |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
Management actions
Implement targeted control of rats and cats in natural areas to reduce predation on the Norfolk Island robin. Restore native forest inside the national park, but also outside the park, with patches of appropriate size, composition, and physical connectedness, to enable passerine birds to expand their range. Conduct weed management (particularly red guava) with planting of native vegetation to avoid habitat becoming unsuitable and to allow ground feeding.
Recovery target
The recovery target is shown in Table 54.
Table 54 Recovery target for Petroica multicolor
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Vulnerable | 750 (700–800) | 59% in the national park 1% in public reserves 40% in other land | The population is at least 1500 individuals and the distribution extends outside the national park and its fringes to other areas of the island (such as other reserves and more southern parts of the island) |
Relevant literature
Bell BD (1990) The status and management of the White-breasted White-eye and other birds of Norfolk Island. Unpublished report to the Australian Nature Conservation Agency.
Bureau of Meteorology (2019) Temperature and rainfall changes at remote Australian Islands and Antarctic sites. Accessed 24 January 2024.
Christian M (2024) personal communication by email, 12 January.
Commonwealth of Australia (2005) National Recovery Plan for the Norfolk Island Scarlet Robin Petroica multicolor multicolor and the Norfolk Island Golden Whistler Pachycephala pectoralis xanthroprocta. Department of the Environment and Heritage, Canberra.
Garnett ST & Baker GB (2021) The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne.
Major R (1989) Reproductive output and recruitment of the Norfolk Island Scarlet Robin (Petroica multicolor multicolor) Phase II. Report to the Australian National Parks and Wildlife Service, Canberra.
Nance AH, Mitchell W, Clarke RH, Wilson M, Brown SM, Macgregor NA, Dutson G & Garnett ST (2021b) Norfolk Island Robin Petroica multicolor, in ST Garnett & GB Baker (eds), The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne. pp. 741-744.
Robinson D (1988) Ecology and Management of the Scarlet Robin, White-breasted White-eye, and Long-billed White-eye of Norfolk Island. Consultants’ report to the Australian National Parks and Wildlife Service, Canberra.
Robinson D (1997) An evaluation of the status of the Norfolk Island Robin following rat-control and weed-control works in the Norfolk Island National Park. Report to Environment Australia, Canberra.
Schodde R, Fullagar P & Hermes N (1983) A review of Norfolk Island birds past and present (Special Publication No. 8). Australian National Parks and Wildlife Service, Canberra.
Zosterops albogularis—white-breasted white-eye, grinnell
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Extinct
Non-statutory Listing Status: Classified as extinct in the Action Plan for Australian Birds 2020 (Garnett & Baker 2021).
Distribution and abundance
Early records suggest the white-breasted white-eye (Zosterops albogularis) was common and widespread on the island before the end of the 1800s, after which the population declined dramatically to an estimate of fewer than 50 birds by 1962 (Schodde et al. 1983). By the 1970s the population had further declined and there have only been scattered sightings over the last two decades including two in 1991, four in 1994 and one in 2000 (Garnett & Crowley 2000).
The last reported confirmed sighting was in 2004 (Christian 2005). While the Action Plan for Australian Birds 2010 (Garnett et al. 2011) assessed the species as Critically Endangered, the more recent assessment is that there was a high probability that the species was already extinct in 2010 and that persistence a decade later is not possible (Clarke et al. 2021).
Ecology
The species was a tree-creeper feeding on small insects in the canopy.
Habitat
The species occurred mainly in native forest that was free of weeds, though there were earlier records of nesting in orchards and red guava. The last sightings were in the national park.
Threats
The decline of this species was probably due primarily to predation by black rats, with additional pressure from clearing of habitat and competition from the self-introduced Australian silvereye (Z. lateralis; Clarke et al. 2021).
Impact on other species
None known.
Management actions
Actions to support other passerines would provide some benefit to this species; however, the species is no longer thought to persist on the island.
Relevant literature
Bell BD (1990) The status and management of the White-breasted White-eye and other birds of Norfolk Island. Unpublished report to the Australian Nature Conservation Agency.
Christian M (2005) Norfolk Island…the birds. Green Eyes Publications, Norfolk Island.
Clarke RH, Dutson G, Olsen P & Garnett ST (2021) White-chested White-eye Zosterops albogularis, in ST Garnett & GB Baker (eds), The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne. pp. 762–763.
Garnett ST & Baker GB (2021) The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne.
Garnett ST & Crowley GM (2000) The Action Plan for Australian Birds. Environment Australia.
Garnett ST, Szabo J & Dutson G (2011) The Action Plan for Australian Birds 2010. CSIRO Publishing, Melbourne.
Robinson D (1988) Ecology and Management of the Scarlet Robin, White-breasted White-eye, and Long-billed White-eye of Norfolk Island. Consultants’ report to the Australian National Parks and Wildlife Service, Canberra.
Schodde R, Fullagar P & Hermes N (1983) A review of Norfolk Island birds past and present (Special Publication No. 8). Australian National Parks and Wildlife Service, Canberra.
Zosterops tenuirostris—slender-billed white-eye
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: None.
Non-statutory Listing Status: Described as Vulnerable in the Action Plan for Australian Birds 2020 (Garnett & Baker 2021).
Distribution and abundance
A moderately abundant species originally derived from the Australian silvereye (Zosterops lateralis). A survey in 2009 estimated there were 4030 individuals (95% CI 2550–6360) in the national park (Dutson 2013). A later survey in 2016 confirmed there to be little change (Director of National Parks unpublished).
This species has gradually disappeared from all parts of the island that have been extensively cleared, a decline that has probably been exacerbated by the arrival of the black rat (Robinson 1997). It is now largely confined to the national park, with birds observed outside of the national park thought to be dispersing individuals.
Ecology
Slender-billed white-eyes forage in small groups and appear to have a different ecological niche to the self-introduced silvereye (Robinson 1988). They have a long down-curved bill and use it to probe fissures in bark for insects. They also eat fruit, including introduced species such as the red guava, with the white-eye likely to disperse its seeds.
Habitat
Slender-billed white-eyes occur primarily in rainforest, rainforest remnants, and tall secondary forest, avoiding lower thickets and garden and forest edges (Schodde et al. 1983).
Threats
The main threat to the species is predation from black rats. Predation by cats and degradation and loss of habitat are additional threats (Nance et al. 2021c). This species has probably managed to survive due to its habit of fast movement and remote nest construction on slender branches which do not support the weight of feral animal predators (Director of National Parks 2010).
Impact on other species
None known.
Management actions
Survey to monitor for any signs of further population decline. Assess the need for listing as a threatened species. Undertake targeted predator control of black rats and feral cats. Restore native forest inside and outside the national park, including management to reduce size of areas dominated by red guava.
Relevant literature
Christian M (2005) Norfolk Island … the birds. Green Eyes Publications, Norfolk Island.
Dutson G (2013) Population densities and conservation status of Norfolk Island forest birds. Bird Conservation International 23, 271–282.
Garnett ST & Baker GB (2021) The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne.
Garnett ST & Crowley GM (2000) The Action Plan for Australian Birds. Environment Australia.
Nance AH, Mitchell W, Clarke R, Wilson M, Brown SM, Macgregor NA, Dutson G & Garnett ST (2021c) Slender-billed White-eye Zosterops tenuirostris, in ST Garnett & GB Baker (eds), The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne. pp. 763-765.
Robinson D (1988) Ecology and Management of the Scarlet Robin, White-breasted White-eye, and Long-billed White-eye of Norfolk Island. Consultants’ report to the Australian National Parks and Wildlife Service, Canberra.
Robinson D (1997) An evaluation of the status of the Norfolk Island Robin following rat-control and weed-control works in the Norfolk Island National Park. Report to Environment Australia, Canberra.
Schodde R, Fullagar P & Hermes N (1983) A review of Norfolk Island birds past and present (Special Publication No. 8). Australian National Parks and Wildlife Service, Canberra.
Anous albivittus albivittus—Tasman grey noddy, grey ternlet (western pacific)
Conservation significance
EPBC Act Listing Status: Not listed.
State Listing Status: Listed as Vulnerable under the Biodiversity Conservation Act 2016 (New South Wales).
Non-statutory Listing Status: Described as near threatened in the Action Plan for Australian Birds 2010 (Garnett et al. 2011).
Distribution
Widespread throughout the subtropical and tropical zones of the Indian and West Pacific Oceans. It breeds on the Norfolk Island Group and in Australia on Ashmore Reef, Rowley Shoals, Cocos Keeling Islands, Christmas Island, islands of the Great Barrier Reef and islands of the Lord Howe Island.
A widespread coastal summer breeding species, this species is well established on the Norfolk Island Group and occurs on all three islands. Numbers appear to have been stable, particularly on Phillip Island where there are no predators (Schodde et al. 1983). Phillip Island supports one of the largest breeding populations in Australia. 100–1000 pairs were estimated during 2006 (Priddel et al. 2010) and <1000 pairs in 2017–18 (Carlile & O’Dwyer 2018).
Ecology
Nest selection occurs in November with eggs laid through December, and eggs hatch up until early February, after 45 days of incubation. Usually a single egg is laid and only one chick is ever brooded, with fledging at 85 days. Nest consists of a scrape on the ground on an inaccessible ledge or under shrubs usually near the tops of coastal sea cliffs or steep hills.
Diet consists of squid and fish taken from far offshore.
Habitat
Marine, pelagic mainly in subtropical and tropical waters.
Threats
The main threat to this species is the reduction in the quality of foraging areas due to climate-related shifts in oceanic resources. On Norfolk Island, other threats include interference by people and predation by cats and rats where ternlets nest above the cliff edge or in accessible areas (such as Hundred Acres Reserve and Rocky Point Reserve). Most of the population is not under threat as they nest below the cliff edge. On Phillip Island, additional threats include reforestation, which could render the internal parts of the island unavailable for nesting, and predation of unattended nestlings by purple swamphens.
Impact on other species
None known.
Management actions
Protect nesting areas on Norfolk Island from disturbance by rats and cats. Continue the control of purple swamphens on Phillip Island. Retain status of pest-free Phillip Island through detection monitoring for introduced vertebrates and invertebrates.
Relevant literature
Carlile N & O’Dwyer T (2018) NI2016–26 Report to the Director national parks and Manager Norfolk Island National Park. Office of Environment and Heritage NSW.
Christian M (2005) Norfolk Island … the birds. Green Eyes Publications, Norfolk Island.
Garnett ST & Crowley GM (2000) The Action Plan for Australian Birds. Environment Australia.
Garnett ST, Szabo J & Dutson G (2011) The Action Plan for Australian Birds 2010. CSIRO Publishing, Melbourne.
Priddel D, Carlile N, Evans O, Evans B & McCoy H (2010) A review of the seabirds of Phillip Island in the Norfolk Island Group. Notornis 57, 113–127.
Schodde R, Fullagar P & Hermes N (1983) A review of Norfolk Island birds past and present (Special Publication No. 8). Australian National Parks and Wildlife Service, Canberra.
Anous minutus—black noddy
Conservation significance
Secure, widespread
EPBC Act Listing Status: Marine
Distribution
Widespread over subtropical and tropical seas worldwide, breeding on various islands. It breeds on the Norfolk Island Group and in Australia on the islands of the Great Barrier Reef, north-west Australia, and Lord Howe Island.
The black noddy (Anous minutus) is the most common of the three noddy species present in the Norfolk Island region. It is a spring and summer breeding species that is well established on Norfolk and Phillip Islands. Large rookeries occur in the tall pines of Hundred Acre Reserve, Titerack Valley (in the national park at the end of McLaughlan’s Lane), above Bloody Bridge and in white oak (Lagunaria patersonia) and Norfolk Island pines on Phillip Island (Christian 2005). In 1977 the breeding population on Phillip Island was estimated at between 1,000 and 10,000 breeding pairs (Fullagar 1978). The number of rookeries has fallen over the past few decades, and 100–1000 pairs were estimated in 2010 (Priddel et al. 2010).
Ecology
Breeding season is from October to March. The black noddy lays one egg and shares incubation for 36 days. Hatching to fledging spans approximately 50 days. Nests built of leaves and twigs cemented with excreta in Norfolk Island pines or white oaks.
Diet consists mainly of fish. Forages typically in flocks, swooping and snatching prey at the surface.
Habitat
Exclusively pelagic mainly in tropical and subtropical waters. Often feeds at sea in groups.
Threats
Main threats to the black noddy are reduction in quality of foraging areas through climate related shifts in oceanic resources, and on Norfolk Island, degradation and loss of habitat in pine forest through cattle grazing, weed invasion and development pressure. Other threats include predation from cats and possibly introduced ants.
Impact on other species
None known.
Management actions
Protect nesting areas and control feral predators around nesting areas on Norfolk Island.
Encourage protection of pine forest habitat through covenants on private land. Depleted colonies in the park should be restored through enhancing pine forest habitat in the north-east corner of the park and The Chord area.
Retain status of pest-free Phillip Island through detection monitoring for introduced vertebrates and invertebrates.
Relevant literature
Christian M (2005) Norfolk Island … the birds. Green Eyes Publications, Norfolk Island.
Fullagar PJ (1978) Norfolk Island birds. Unpublished report to RAOU Congress, Norfolk Island.
Priddel D, Carlile N, Evans O, Evans B & McCoy H (2010) A review of the seabirds of Phillip Island in the Norfolk Island Group. Notornis 57, 113–127.
Schodde R, Fullagar P & Hermes N (1983) A review of Norfolk Island birds past and present (Special Publication No. 8). Australian National Parks and Wildlife Service, Canberra.
Anous stolidus—common noddy
Conservation significance
Secure, widespread.
EPBC Act Listing Status: Marine, Migratory.
Distribution
Widespread over subtropical and tropical seas worldwide, breeding on various islands. It breeds on the Norfolk Island Group and in Australia on the islands of the Great Barrier Reef, north-west Australia, and Lord Howe Island.
The common noddy is a common spring and summer breeding species that gathers on the islands to nest, then disperses out to sea. It nests in small groups on the ground or amongst rocks on Phillip Island. Estimates of breeding population on Phillip Island range from >1000 pairs in 1977 (Fullagar 1978) to a few hundred in 1978–79 (Tarburton 1981; Schodde et al. 1983) and several hundred pairs in 1985 (Hermes et al. 1986).
Ecology
Breeds on Phillip Island in spring and early summer (October to December–January). On Norfolk Island, each pair raises a single brood each year. The single egg can be replaced if lost and is incubated for 35 days. Hatching to fledging spans 50 days. Nests in a depression on the ground or in rocks. Feeds mainly on fish, foraging typically in flocks and swooping to take prey from the sea surface.
Habitat
Marine, pelagic mainly in tropical or subtropical waters.
Threats
The main threat to the black noddy is the reduction in the quality of foraging areas through climate‑related shifts in oceanic resources. As a ground nesting species, the presence of cats on Norfolk Island probably excludes it from breeding there. This species is largely secure in Australasia, but predation from cats and disturbance from humans have adversely affected some populations.
Impact on other species
None known.
Management actions
Protect and enhance nesting areas through revegetation efforts. Retain status of pest-free Phillip Island through detection monitoring for introduced vertebrates and invertebrates.
Relevant literature
Christian M (2005) Norfolk Island … the birds. Green Eyes Publications, Norfolk Island.
Fullagar PJ (1978) Norfolk Island birds. Unpublished report to RAOU Congress, Norfolk Island.
Hermes N, Evans O & Evans B (1986) Norfolk Island birds: a review 1985. Notornis 33, 141–149.
Schodde R, Fullagar P & Hermes N (1983) A review of Norfolk Island birds past and present (Special Publication No. 8). Australian National Parks and Wildlife Service, Canberra.
Tarburton MK (1981) Seabirds nesting on Norfolk Island. Notornis 28, 209–211.
Ardenna carneipes—flesh-footed shearwater
Conservation significance
EPBC Act Listing Status: Marine, Migratory (listed marine under the EPBC Act as Puffinus carneipes).
State Listing Status: Listed as Vulnerable under the Biodiversity Conservation Act 2016 (NSW).
Non-statutory Listing Status: Listed as Near Threatened in The Action Plan for Australian Birds 2020 (Garnett & Baker 2021).
Distribution
A widespread species across the southern Indian Ocean and south-eastern Pacific Ocean; breeding and non-breeding visitor to the coastal and pelagic waters of southern Australia.
Priddel et al. (2010) estimated the population of flesh-footed shearwater on Phillip Island to be 1–10 pairs. Breeding here was confirmed in 2011 when a fledgling was intercepted at the known breeding site below Red Knoll (Carlile 2011).
Ecology
Breeds late August to May. Eggs are incubated for less than 60 days, and young fledge approximately three months after hatching. Nests in a deep burrow.
Diet consists of small fish and squid. Food captured by diving and pursuit plunging to 10 m and by surface seizing.
Habitat
Breeding may occur on islands within the Australasian region and Indian Ocean. Nests are made in burrows on gentle to steep slopes where burrowing is not restricted by dense vegetation, deep litter or bare rock. Nesting colonies require clear, elevated places to allow sufficient space for take-off.
Threats
The main threat to the flesh-footed shearwater is the reduction in the quality of foraging areas through climate-related shifts in oceanic resources. The species is also threatened by degradation and loss of breeding habitat. Offshore windfarms along the east coast of Australia may represent an emerging threat due to turbine strike.
Impact on other species
None known.
Management actions
Retain status of pest-free Phillip Island through detection monitoring for introduced vertebrates and invertebrates. Protect nesting areas and conduct surveillance camera monitoring of known burrows between December and May annually to detect continued breeding below Red Knoll.
Relevant literature
Carlile N (2011) Observations of seabirds on Phillip Island 8–12 May 2011. Unpublished report.
Christian M (2005) Norfolk Island … the birds. Green Eyes Publications, Norfolk Island.
Garnett ST & Baker GB (2021) The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne.
Garnett ST, Szabo J & Dutson G (2011) The Action Plan for Australian Birds 2010. CSIRO Publishing, Melbourne.
Priddel D, Carlile N, Evans O, Evans B & McCoy H (2010) A review of the seabirds of Phillip Island in the Norfolk Island Group. Notornis 57, 113–127.
Ardenna pacifica—wedge-tailed shearwater, ghost bird
Conservation significance
EPBC Act Listing Status: Marine, migratory (listed marine under the EPBC Act as Puffinus pacificus)
Non-statutory Listing Status: Listed as Least Concern in the Action Plan for Australian Birds 2010 (Garnett et al. 2011)
Distribution
This species is the most common and widespread shearwater in the south-west Pacific and Indian Oceans with many breeding localities. It is a common breeding summer migrant to the Norfolk Island Group where it breeds on all three islands (Schodde et al. 1983) with a total breeding population of several hundreds of thousands of birds (Tarburton 1981). The Phillip Island population was estimated in 2006 as between 1,000–10,000 pairs (Priddel et al. 2010). Black rats have caused populations to decline on some Pacific islands (such as Midway), and feral cats severely damage shearwater colonies (Fitzherbert & Peter 1988).
Ecology
Summer breeder returning to Norfolk Island in October and departing in May. Incubation is 53 days of their single egg and hatching to fledging takes approximately three months. On Norfolk Island the nests are in crowded colonies often concentrated among tussocks of kikuyu (Cenchrus clandestinus). On Phillip Island they are amongst other burrowing species in deeper soils. The nest is at the end of a burrow that can be 1–2 m long.
Diet consists of squid, fish and crustaceans, caught by lunge-diving to two metres.
Habitat
Marine, pelagic mainly in tropical and subtropical waters. Feeds at sea during the day; rafts of birds can often be seen just offshore before they return to the breeding colonies at dusk.
Threats
The main threats to the wedge-tailed shearwater are the reduction in the quality of foraging areas through climate-related shifts in oceanic resources, predation from cats, and degradation and loss of breeding habitat, particularly through weed invasion from kikuyu. Adults and fledglings can get entangled in kikuyu runners. Offshore windfarms along the east coast of Australia may represent an emerging threat due to turbine strike.
Impact on other species
In limited areas on Phillip Island, when adults return to breed, they will evict unfledged providence petrel (Pterodroma solandri) chicks from their burrows.
Management actions
Protect nesting areas through appropriate weed management.
Relevant literature
Christian M (2005) Norfolk Island … the birds. Green Eyes Publications, Norfolk Island.
Garnett ST, Szabo J & Dutson G (2011) The Action Plan for Australian Birds 2010. CSIRO Publishing, Melbourne.
Fitzherbert K & Peter J (1988) Status and movement of Australian migratory birds Vol 1. Procellariiformes Part II. Royal Australasian Ornithologists Union.
Priddel D, Carlile N, Evans O, Evans B & McCoy H (2010) A review of the seabirds of Phillip Island in the Norfolk Island Group. Notornis 57, 113–127.
Schodde R, Fullagar P & Hermes N (1983) A review of Norfolk Island birds past and present (Special Publication No. 8). Australian National Parks and Wildlife Service, Canberra.
Tarburton MK (1981) Seabirds nesting on Norfolk Island. Notornis 28, 209–211.
Fregetta grallaria grallaria—Tasman white-bellied storm-petrel
Conservation significance
EPBC Act Listing Status: Vulnerable, Marine
Non-statutory Listing Status: Described as Vulnerable in the Action Plan for Australian Birds 2020 (Garnett and Baker 2021).
Distribution and abundance
Breeding has been recorded on islands and islets off the east coast of New South Wales (such as Muttonbird Island), Ball’s Pyramid in the Lord Howe Island group, and Macauley and Curtis Islands in the Kermadec Island Group. It is also thought to breed on Phillip Island in the Norfolk Island Group. Fewer than 100 pairs are thought to nest on islands off the New South Wales coast, and fewer than 700 in the Kermadec Islands. Numbers on Ball’s Pyramid are unknown (Garnett and Baker 2021.) It migrates between its breeding locations and its non-breeding grounds in the Tasman Sea, Coral Sea and central Pacific Ocean.
Ecology
Breeds late summer to autumn. Eggs are laid from January to March, and young fledge in May. Nests in crevices between large volcanic rocks and in burrows excavated in banks. Clutches consist of a single egg, which is incubated by both parents for approximately 37 days.
Forages both at day and at night on small crustaceans and squid, usually far from shore, by skimming low over the ocean and plucking prey from beneath the surface of the water.
Habitat
Marine, highly pelagic across sub-tropical and tropical waters in the Tasman Sea, Coral Sea and the central Pacific Ocean, and rarely approaches land except to return to colonies.
Threats
The main known threats are predation from invasive species, particular the black rat. It is thought the population on Lord Howe Island was extirpated by black rats after they arrived in 1918.
Impact on other species
None known.
Management actions
Maintain biosecurity measures on breeding islands and islets, particularly to prevent the arrival of rats or cats. Confirm rodent eradication on Lord Howe Island and encourage chances of re‑establishment of the species there (Garnett and Baker 2021).
Relevant literature
Garnett ST & Baker GB (2021) The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne.
Gygis alba—white tern
Conservation significance
EPBC Act Listing Status: Marine
Non-statutory Listing Status: Described as vulnerable in the Action Plan for Australian Birds (Garnett & Crowley 2000).
Distribution and abundance
This species breeds on tropical and subtropical islands throughout the Pacific, Indian, and South Atlantic oceans. Within Australasian waters it breeds on Norfolk Island, Lord Howe Island and the in the Cocos Keeling group. On Norfolk Island, white oak (Lagunaria patersonia) and Norfolk Island pine (Araucaria heterophylla) are the favoured nest trees (Schodde et al. 1983). The north-west coast of the island supports large rookeries as does the valley behind Bloody Bridge (Christian 2005). There are about 2,000 to 2,500 breeding pairs on Norfolk Island and this species has persisted despite predation from nankeen kestrels (Falco cenchoides) and marsh harriers (Circus approximans) (Garnett & Crowley 2000).
Ecology
A spring/summer breeder with egg laying beginning in October. One egg is laid. No nest is built. The single egg is laid in a notch or depression on the horizontal branch of a tree. Its diet consists mainly of small fish and squid. Feeds mainly by surface seizing and often feeds in small groups. At sea tracking reveals that little time is spent on the ocean surface annually (Carlile & O’Dwyer 2022).
Habitat
Marine, pelagic mostly in tropical and subtropical waters. The Lord Howe population shows significant non-breeding movements into the Coral Sea (Carlile & O’Dwyer 2022).
Threats
The main threats are predation from cats, degradation and loss of habitat and increased frequencies of intense storms resulting in nest failure.
Impact on other species
None known.
Management actions
Protection of nesting areas, and regular monitoring of the population to detect significant changes. Restore remnant coastal pine/oak forests in the national park and in coastal reserves.
Relevant literature
Carlile N & O’Dwyer T (2022) At-sea movements of the White Tern Gygis alba in waters off Eastern Australia. Marine Ornithology 50, 157–164.
Christian M (2005) Norfolk Island …the birds. Green Eyes Publications, Norfolk Island.
Garnett ST & Crowley GM (2000) The Action Plan for Australian Birds. Environment Australia.
Schodde R, Fullagar P & Hermes N (1983) A review of Norfolk Island birds past and present (Special Publication No. 8). Australian National Parks and Wildlife Service, Canberra.
Morus serrator—Australasian gannet
Conservation significance
EPBC Act Listing Status: Marine.
Non-statutory Listing Status: Described as least concern by the Action Plan for Australian Birds 2010 (Garnett et al. 2011).
Distribution
The species is largely found in temperate waters with breeding colonies on rocky islands off Victoria, Tasmania and the North Island of New Zealand.
From the 1960s to the 2000s, the Australasian gannet was a rare summer breeding species in the Norfolk Island Group. The species was first recorded nesting on Nepean Island in 1961, then shifted to Phillip Island, with up to four pairs reported (Tarburton 1981). In 2005, only three pairs were known to nest on Phillip Island (Christian 2005). By 2006, two pairs were present (Priddel et al. 2010) and since 2011 no breeding has been observed (N Carlile 2024. pers comm 12 January). Recently, the species has been recorded on one of the offshore stacks north of Norfolk Island (M Christian 2024. pers comm 12 January), with possible breeding not yet investigated.
Ecology
Breeding: formerly bred on Phillip Island in summer.
Nesting: Nest colonially on mounds of guano mixed with seaweed or earth built on rocks.
Foraging: Feeds on small fish and cephalopods.
Habitat
A marine pelagic species whose non-breeding range extends from the seas off southern Australia to northern Queensland and the Lord Howe and the Norfolk Island Groups. Juveniles may remain near breeding colonies throughout the year although most non-breeding birds disperse.
Threats
The main threats to the Australasian gannet include changes in the marine environment, entanglement in long-line fishing gear, and competition from the fishing industry for oceanic resources. Both on Phillip Island and on Nepean Island, breeding birds are free from predation from introduced rats and cats.
Impact on other species
None known.
Management actions
None required until re-nesting is detected.
Relevant literature
Carlile N (2024) Personal communication by email, 12 January.
Christian M (2005) Norfolk Island … the birds. Green Eyes Publications, Norfolk Island.
Christian M (2024) Personal communication by email, 12 January.
Garnett ST & Crowley GM (2000) The Action Plan for Australian Birds. Environment Australia, Canberra.
Garnett ST, Szabo J & Dutson G (2011) The Action Plan for Australian Birds 2010. CSIRO Publishing, Melbourne.
Priddel D, Carlile N, Evans O, Evans B & McCoy H (2010) A review of the seabirds of Phillip Island in the Norfolk Island Group. Notornis 57, 113–127.
Tarburton MK (1981) Seabirds nesting on Norfolk Island. Notornis 28, 209–211.
Onychoprion fuscata—sooty tern, whale bird
Conservation significance
EPBC Act Listing Status: Marine (listed marine under the EPBC Act as Sterna fuscata)
State Listing Status: Listed as Vulnerable under the Biodiversity Conservation Act 2016 (NSW).
Distribution
A wide distribution over tropical and subtropical seas, breeding on numerous islands (including islands of the Great Barrier Reef) in north-west Australia and the south-west Pacific (including the Lord Howe and Norfolk Island Groups). Preliminary findings from tracking of sooty terns on Phillip Island indicate that during the breeding season the species forages within approximately 600 km of Phillip Island (Gorta unpublished data 2023). In the non-breeding season, which began as early as mid‑January 2023 in the 2022–23 season, sooty terns largely migrated into the South and West Pacific (Gorta unpublished data 2023).
Known on Norfolk Island as the whale bird, this is an abundant summer breeding species that nests on Phillip and Nepean Islands and on the north coast of the main island (Schodde et al. 1983). There were an estimated 80,000 to 140,000 birds breeding in the Norfolk Island Group (Blakers et al. 1984) including about 20,000 on Phillip Island and several hundred on Nepean Island (Fullagar 1978). In 2006 the population here was estimated to be between 1000–10,000 pairs (Priddel et al. 2010). Breeding was significantly reduced on Phillip Island by 2016 (fewer than 1,000 pairs) and only recommenced in larger numbers following purple swamphen control in 2019 (Carlile & O’Dwyer 2023). Preliminary results from surveys during the 2022–23 breeding season indicate the current breeding population on Phillip Island numbers from 6,000-8,000 pairs (Gorta unpublished data 2023).
In 1908, 10,000 to 15,000 eggs were harvested from Nepean Island several times a week (Schodde et al. 1983). This species is subject to a limited annual open season for the harvesting of eggs.
Ecology
Present around Norfolk Island from August, this species is a spring/summer breeder with most pairs starting to nest in November, but the laying season is prolonged by the harvesting of eggs by islanders. A single egg is laid, which can be replaced after roughly 2 weeks. Incubation lasts around 28 days and hatching to fledging spans approximately 50 days. Nest is a shallow scrape in sand or soft soil. Will nest in open areas, but often partially or completely underneath shrubby vegetation on Phillip Island.
Forages nocturnally and diurnally by swooping to snatch pelagic squid, crustaceans and fish at the ocean surface.
Habitat
Marine, pelagic in tropical and subtropical waters, breeding on islands.
Threats
The main threat to the sooty tern is the reduction in the quality of foraging areas through climate related shifts in oceanic resources. Additional threats include predation by rats and cats on Norfolk Island and predation of nests by swamphens on Phillip Island. Loss of habitat to significant revegetation can limit areas of breeding. Offshore windfarms along the east coast of Australia may represent an emerging threat due to turbine strike.
Impact on other species
None known.
Risk assessment
The risk assessment is shown in Table 55.
Table 55 Risk assessment for Onychoprion fuscata
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Likely (51–90%) | Major | High |
2. Loss and fragmentation of native vegetation through current or future land clearing | Unlikely (11–25%) | Minor | Low |
3. Degradation of native vegetation through past grazing or loss of nutrients | Likely (51–90%) | Major | High |
4. Degradation of native vegetation through current or future grazing | Unlikely (11–25%) | Negligible | Negligible |
5. Lack of available nest sites | Rare (0–10%) | Negligible | Negligible |
6. Predation by rodents | Possible (26–50%) | Minor | Low |
7. Predation by cats | Unlikely (11–25%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Unknown | Unknown |
9. Predation by swamphens | Almost certain (91–100%) | Major | Extreme |
10. Predation by Argentine ant | Possible (26–50%) | Major | High |
11. Competition from/change of habitat because of weed invasion | Unlikely (11–25%) | Minor | Low |
12. Infection by pathogens already present | Unlikely (11–25%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Possible (26–50%) | Unknown | Unknown |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Likely (51–90%) | Major | High |
15. Problems caused by small populations, including lack of genetic diversity | Unlikely (11–25%) | Minor | Low |
Management actions
Continue control of swamphens on Phillip Island and experiment with new control approaches. Protect nesting areas from woody weed invasion. Retain status of pest-free Phillip Island through detection monitoring for introduced vertebrates and invertebrates. Use drone mapping of colony extent on Phillip Island annually in December as a proxy to detect significant changes in populations.
Relevant literature
Blakers M, Davies SJJJF & Reilly PM (1984) An atlas of Australian birds. Royal Australasian Ornithologists Union, Melbourne University Press, Melbourne.
Carlile N & O’Dwyer T (2023) Conservation of the surface-nesting Kermadec Petrel Pterodroma neglecta neglecta in the South Pacific: Clarifying breeding ecology and the threat of avian ground predators. Bird Conservation International 33, e44, 1–9.
Christian M (2005) Norfolk Island … the birds. Green Eyes Publications, Norfolk Island.
Feare CJ (1976) The breeding of the Sooty Tern Sterna fuscata in the Seychelles and the effects of experimental removal of its eggs. Journal of Zoology 179(3), 317–360.
Fullagar PJ (1978) Norfolk Island birds. Unpublished report to RAOU Congress, Norfolk Island.
O'Neill L (2006) The breeding and feeding ecology of the Sooty Tern Sterna fuscata on Lord Howe Island. PhD Thesis, Charles Sturt University, Albury, NSW.
Priddel D, Carlile N, Evans O, Evans B & McCoy H (2010) A review of the seabirds of Phillip Island in the Norfolk Island Group. Notornis 57, 113–127.
Schodde R, Fullagar P & Hermes N (1983) A review of Norfolk Island birds past and present (Special Publication No. 8). Australian National Parks and Wildlife Service, Canberra.
Phaethon rubricauda—red-tailed tropicbird
Conservation significance
EPBC Act Listing Status: Marine, Migratory.
State Listing Status: Listed as Vulnerable under the Biodiversity Conservation Act 2016 (New South Wales).
Non-statutory Listing Status: Described as near threatened in the Action Plan for Australian Birds 2010 (Garnett et al. 2011).
Distribution
Widespread throughout the subtropical and tropical zones of the Indian and West Pacific Oceans. In Australia it breeds on Ashmore Reef, Rowley Shoals, Cocos-Keeling Islands, Christmas Island, islands of the Great Barrier Reef and islands of the Lord Howe Island and Norfolk Island Group.
A widespread coastal summer breeding species, this species is well established on the Norfolk Island Group and occurs on all three islands. Numbers were apparently stable, particularly on Phillip Island where there are no predators (Schodde et al. 1983). Phillip Island supports one of the largest breeding populations in Australia. 100–1000 pairs were estimated during 2006 (Priddel et al. 2010) and <1000 pairs in 2017–18 (Carlile & O’Dwyer 2018).
Ecology
Nest selection occurs in November with eggs laid through December, and eggs hatch into early February after 45 days of incubation. Usually a single egg is laid and only one chick is ever brooded, with fledging at 85 days. Nest consists of a scrape on the ground on an inaccessible ledge or under shrubs usually near the tops of coastal sea cliffs or steep hills or on plateaus.
Diet consists of squid and fish taken far from shore.
Habitat
Marine, pelagic mainly in subtropical and tropical waters.
Threats
The main threat to the red-tailed tropicbird is reduction in the quality of foraging areas through climate-related shifts in oceanic resources. On Norfolk Island, threats include interference by people and predation by cats and rats where tropicbirds nest above the cliff edge or in accessible areas (such as Hundred Acres Reserve and Rocky Point Reserve). Most of the population is not under threat as they nest below the cliff edge. On Phillip Island complete reforestation would render the internal parts of the island unavailable for nesting and increase predation of unattended nestlings by purple swamphen.
Impact on other species
Excludes Kermadec petrel from nest sites on Phillip Island, which has led to failed nesting attempts for the petrel (Carlile & O’Dwyer 2023).
Management actions
Protect nesting areas on Norfolk Island from disturbance by rats and cats. Continue the control of swamphens on Phillip Island. Retain status of pest-free Phillip Island through detection monitoring for introduced vertebrates and invertebrates.
Relevant literature
Carlile N & O’Dwyer T (2018) NI2016–26 Report to the Director national parks and Manager Norfolk Island National Park. Office of Environment and Heritage NSW.
Christian M (2005) Norfolk Island … the birds. Green Eyes Publications, Norfolk Island.
Garnett ST & Crowley GM (2000) The Action Plan for Australian Birds. Environment Australia, Canberra.
Garnett ST, Szabo J & Dutson G (2011) The Action Plan for Australian Birds 2010. CSIRO Publishing, Melbourne.
Priddel D, Carlile N, Evans O, Evans B & McCoy H (2010) A review of the seabirds of Phillip Island in the Norfolk Island Group. Notornis 57, 113–127.
Schodde R, Fullagar P & Hermes N (1983) A review of Norfolk Island birds past and present (Special Publication No. 8). Australian National Parks and Wildlife Service, Canberra.
Pterodroma cervicalis—white-necked petrel
Conservation significance
EPBC Act Listing Status: Marine.
Non-statutory Listing Status: The Australian breeding population is described as Endangered in the Action Plan for Australian Birds 2020 (Garnett & Baker 2021).
Distribution
Before 1991, the white-necked petrel (Pterodroma cervicalis) was only known to breed at Macauley Island (Tennyson et al. 1989) and Raoul Island (Iredale 1910) in the Kermadec Group, with the latter population going extinct after the establishment of rodents there. A pair were found breeding on Phillip Island in 1992 (Priddel et al. 2010). This increased to about 10 breeding birds in 1995 (Garnett & Crowley 2000). There were two colonies each consisting of 10–12 breeding pairs on Phillip Island in 2010 (Director of National Parks 2010), 10–100 pairs in 2006 (Priddel et al. 2010) and 44 pairs in 2017–18 (Carlile & O’Dwyer 2018). Prior to purple swamphen management their population likely suffered from predation of eggs and chicks by that species (Halpin et al. 2021).
Ecology
Breeds in summer, arriving in late November and departing the island the following May. Incubation period is unknown but hatching to fledging spans over three months. Nests in burrows or on the surface under natural and artificial cover.
Feeds on small squid and crustaceans taken from the open ocean (Halpin et al. 2022). Not known to frequent at-sea areas of high plastic concentrations in breeding or non-breeding periods (Clarke et al. 2023).
Habitat
Marine pelagic species that migrates to the North Pacific when not breeding.
Threats
The main threats to the white-necked petrel are reduction in the quality of foraging areas through climate-related shifts in oceanic resources, predation of chicks by purple swamphens, human disturbance of nests, and degradation and loss of breeding habitat.
Impact on other species
None known.
Risk assessment
The risk assessment is shown in Table 56 and Table 57.
Table 56 Risk assessment for Pterodroma cervicalis (current range, Phillip Island)
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Unlikely (11–25%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Likely (51–90%) | Major | High |
4. Degradation of native vegetation through current or future grazing | Unlikely (11–25%) | Negligible | Negligible |
5. Lack of available nest sites | Unlikely (11–25%) | Negligible | Negligible |
6. Predation by rodents | Possible (26–50%) | Extreme | High |
7. Predation by cats | Possible (26–50%) | Extreme | High |
8. Predation or damage by chickens | Unlikely (11–25%) | Negligible | Negligible |
9. Predation by swamphens | Likely (51–90%) | Moderate | Medium |
10. Predation by Argentine ant | Likely (51–90%) | Major | High |
11. Competition from/change of habitat because of weed invasion | Unlikely (11–25%) | Minor | Low |
12. Infection by pathogens already present | Unlikely (11–25%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Possible (26–50%) | Unknown | Unknown |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Likely (51–90%) | Major | High |
15. Problems caused by small populations, including lack of genetic diversity | Likely (51–90%) | Moderate | Medium |
Table 57 Risk assessment for Pterodroma cervicalis (if range expanded to include Norfolk Island)
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Unlikely (11–25%) | Minor | Low |
2. Loss and fragmentation of native vegetation through current or future land clearing | Unlikely (11–25%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Likely (51–90%) | Major | High |
4. Degradation of native vegetation through current or future grazing | Unlikely (11–25%) | Negligible | Negligible |
5. Lack of available nest sites | Possible (26–50%) | Minor | Low |
6. Predation by rodents | Almost certain (91–100%) | Extreme | Extreme |
7. Predation by cats | Almost certain (91–100%) | Extreme | Extreme |
8. Predation or damage by chickens | Unlikely (11–25%) | Negligible | Negligible |
9. Predation by swamphens | Likely (51–90%) | Moderate | Medium |
10. Predation by Argentine ant | Likely (51–90%) | Major | High |
11. Competition from/change of habitat because of weed invasion | Unlikely (11–25%) | Minor | Low |
12. Infection by pathogens already present | Unlikely (11–25%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Possible (26–50%) | Unknown | Unknown |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Likely (51–90%) | Major | High |
15. Problems caused by small populations, including lack of genetic diversity | Likely (51–90%) | Moderate | Medium |
Management actions
Continue control of swamphens on Phillip Island. Retain status of pest-free Phillip Island through detection monitoring for introduced vertebrates and invertebrates. Maintain existing artificial breeding structures where standing camps have been removed and construct additional artificial breeding structures to improve breeding success. Protect natural nesting areas including appropriate weed control and revegetation.
Conduct annual monitoring of a subset (≥ 30) of known nesting sites (both natural and artificial) to provide a measure of breeding success. Nesting sites should be monitored in mid-January for birds incubating eggs and again rechecked in mid-April for fledglings. Near-fledged birds should be banded with Australian Bird and Bat Banding Scheme (ABBBS) bands. Nest surveys should be undertaken every three years for five days in January with a minimum of three nights of four-hour searches, to provide an estimate of population size and colony expansion.
Relevant literature
Carlile N & O’Dwyer T (2018) NI2016–26 Report to the Director national parks and Manager Norfolk Island National Park. Office of Environment and Heritage NSW.
Christian M (2005) Norfolk Island … the birds. Green Eyes Publications, Norfolk Island.
Clark BL, Carneiro APB, Pearmain EJ et al. (2023) Global assessment of marine plastic exposure risk for oceanic birds. Nature Communications 14, 3665.
Director of National Parks (2010) Norfolk Island Region Threatened Species Recovery Plan. Department of the Environment, Water, Heritage and the Arts, Canberra.
Garnett ST & Baker GB (2021) The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne.
Garnett ST & Crowley GM (2000) The Action Plan for Australian Birds. Environment Australia, Canberra.
Halpin LR, Carlile N, Baker GB & Garnett ST (2021) White-necked Petrel Pterodroma cervicalis cervicalis, in ST Garnett & GB Baker (eds), The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne. pp. 177-179.
Halpin LR, Mott R, Clay TA, Humphries GRW, Chatwin TA, Carlile N & Clarke RH (2022) Predicting the foraging habitats of sympatrically breeding gadfly petrels in the South Pacific Ocean. Frontiers in Marine Science 9, 853104.
Iredale T (1910) Bird life on the Kermadec Islands. Emu 10, 2–16.
Priddel D, Carlile N, Evans O, Evans B & McCoy H (2010) A review of the seabirds of Phillip Island in the Norfolk Island Group. Notornis 57, 113–127.
Schodde R, Fullagar P & Hermes N (1983) A review of Norfolk Island birds past and present (Special Publication No. 8). Australian National Parks and Wildlife Service, Canberra.
Tennyson AJD, Taylor GA & Scofield RP (1989) Another visit to Macauley Island. Ornithological Society of New Zealand News 52, 4–5. Supplement to Notornis 36.
Pterodroma neglecta neglecta—Kermadec petrel (western)
Conservation significance
EPBC Act Listing Status: Vulnerable
State Listing Status: Listed as Vulnerable under the Biodiversity Conservation Act 2016 (NSW)
Non-statutory Listing Status: The Australian breeding population is described as Vulnerable in the Action Plan for Australian Birds 2020 (a downlisting of one level since 2010 based on new information rather than a genuine sufficient change in population), with the population visiting Australian territory rated as being of ‘least concern’ (Garnett & Baker 2021).
For further information on the species outside of the Norfolk Island Group, see the species profile on SPRAT.
Distribution
The western subspecies of the Kermadec petrel breeds on islands across the Pacific Ocean as far east as Easter Island, with hybrids on Round Island near Mauritius in the Indian Ocean; however, its current breeding range is smaller than it once was. It nests on the ground so is particularly vulnerable to predation (Merton 1970)—on Raoul Island in the Kermadec Group it is estimated that 250,000 pairs were destroyed by rats between 1910 and 1970. In the Australian region, small numbers of pairs nest on Phillip Island and Ball’s Pyramid to the south of Lord Howe Island. Black rats caused its extinction from Lord Howe Island (Fullagar & Disney 1975) where it was formally probably widespread before the arrival of pigs in 1788. Rats probably prevent it from colonising Norfolk Island (Garnett & Crowley 2000).
The latest estimate of the population on Phillip Island is 20–25 breeding pairs in 2017–18 (Carlile & O’Dwyer 2018) and over 50 pairs in 2019 (Carlile et al. 2021). The small population on Phillip Island remains the most accessible internationally for the study of this species.
The Norfolk Island Group distribution of nesting Kermadec petrels is shown in Map 22.
Ecology
Breeding occurs on Phillip Island in all months of the year with peaks in numbers in spring and summer (Carlile et al. 2021). A single egg is incubated for 50 days, and fledging takes another 96 days (Carlile and O’Dwyer 2023). Nests among rocks and vegetation, under stands of wind-swept African olive (Olea europaea cuspidata) and under clumps of New Zealand flax (Phormium tenax).
Forages far out to sea and feeds on small squid and crustaceans (Halpin et al. 2022). Not known to frequent at-sea areas of high plastic concentrations in breeding or non-breeding periods (Clarke et al. 2023).
Habitat
Marine, pelagic in waters 15-25 degrees C. Breeds on high islands among rocks and vegetation. On Phillip Island, breeding habitat occurs on sloping terrain 182–228 m above the shoreline and up to 85 m from the coast in small sub-colonies under low scrubby woodland (Carlile and O’Dwyer 2023).
Map 22 Distribution of Pterodroma neglecta neglecta within the Norfolk Island Group
Green shading indicates locations where the species has been recorded breeding. Source: Carlile and O’Dwyer 2023.
Threats
Kermadec petrels are prone to nest predation because they nest on the surface of the ground. Purple swamphens prey on eggs and chicks on Phillip Island and are the major immediate threat to the population. Rats and cats have reduced or eliminated Kermadec petrel populations on other islands, and the accidental introduction of either species to Phillip Island would present an extreme threat. Other threats include degradation of breeding habitat, which could occur through revegetation activities either removing excessive African olive or failing to maintain open areas without shrubs near current nesting sites, and the reduction in the quality of foraging areas through climate-related shifts in oceanic resources.
Impact on other species
None known.
Risk assessment
The risk assessment is shown in Table 58 and Table 59.
Table 58 Risk assessment for Pterodroma neglecta neglecta (current range, Phillip Island)
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Unlikely (11–25%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Likely (51–90%) | Major | High |
4. Degradation of native vegetation through current or future grazing | Unlikely (11–25%) | Negligible | Negligible |
5. Lack of available nest sites | Unlikely (11–25%) | Negligible | Negligible |
6. Predation by rodents | Possible (26–50%) | Extreme | High |
7. Predation by cats | Unlikely (11–25%) | Extreme | Medium |
8. Predation or damage by chickens | Rare (0–10%) | n/a | n/a |
9. Predation by swamphens | Likely (51–90%) | Extreme | Extreme |
10. Predation by Argentine ant | Likely (51–90%) | Major | High |
11. Competition from/change of habitat because of weed invasion | Unlikely (11–25%) | Minor | Low |
12. Infection by pathogens already present | Unlikely (11–25%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Possible (26–50%) | Unknown | Unknown |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Likely (51–90%) | Major | High |
15. Problems caused by small populations, including lack of genetic diversity | Likely (51–90%) | Moderate | Medium |
Table 59 Risk assessment for Pterodroma neglecta neglecta (if range expanded to include Norfolk Island)
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Unlikely (11–25%) | Minor | Low |
2. Loss and fragmentation of native vegetation through current or future land clearing | Unlikely (11–25%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Unlikely (11–25%) | Negligible | Negligible |
4. Degradation of native vegetation through current or future grazing | Unlikely (11–25%) | Negligible | Negligible |
5. Lack of available nest sites | Unlikely (11–25%) | Negligible | Negligible |
6. Predation by rodents | Almost certain (91–100%) | Extreme | Extreme |
7. Predation by cats | Almost certain (91–100%) | Extreme | Extreme |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Likely (51–90%) | Major | High |
10. Predation by Argentine ant | Likely (51–90%) | Major | High |
11. Competition from/change of habitat because of weed invasion | Unlikely (11–25%) | Minor | Low |
12. Infection by pathogens already present | Unlikely (11–25%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Possible (26–50%) | Unknown | Unknown |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Likely (51–90%) | Major | High |
15. Problems caused by small populations, including lack of genetic diversity | Likely (51–90%) | Moderate | Medium |
Management actions
Continue control of swamphens on Phillip Island, including maintaining the shooting regime on Phillip Island that was implemented in 2020–21 to reduce nest predation. Protect nesting areas through appropriate weed control and revegetation. Undertake removal and replacement of decrepit nesting structures for seabirds on Phillip Island. Retain status of pest-free Phillip Island through implementation of comprehensive biosecurity procedures (quarantine, surveillance and incursion readiness and response).
Conduct annual monitoring of a subset (≥ 30) of known nesting sites during four targeted site visits annually to provide a measure of breeding success. Every three years, conduct bi-monthly nest monitoring, over a minimum of three nights of four-hour search sessions, to provide an estimate of population size and colony expansion. Near-fledged birds should be banded with ABBBS bands.
The Lord Howe Island Biodiversity Management Plan covers the recovery needs of this species on the Lord Howe Island group. Possible future actions may need to be undertaken in collaboration with the NSW Government as appropriate.
Recovery target
The recovery target is shown in Table 60.
Table 60 Recovery target for Pterodroma neglecta neglecta
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Vulnerable | 150 on Phillip Island (50 breeding pairs) | 100% within the national park | There are at least 100 breeding pairs on Phillip Island with maintained high breeding success |
Relevant literature
Carlile N & O’Dwyer T (2018) NI2016–26 Report to the Director national parks and Manager Norfolk Island National Park. Office of Environment and Heritage NSW.
Carlile N & O’Dwyer T (2023) Conservation of the surface-nesting Kermadec Petrel Pterodroma neglecta neglecta in the South Pacific: Clarifying breeding ecology and the threat of avian ground predators. Bird Conservation International 33, e44, 1–9.
Carlile N, O'Dwyer T, Wilson M, Clarke RH, Brown SM, Baker GB & Garnett ST (2021) Western Kermadec petrel Pterodroma neglecta neglecta, in ST Garnett & GB Baker (eds), The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne. pp. 169-172.
Christian M (2005) Norfolk Island … the birds. Green Eyes Publications, Norfolk Island.
Clark BL, Carneiro APB, Pearmain EJ et al. (2023) Global assessment of marine plastic exposure risk for oceanic birds. Nature Communications 14, 3665.
Fullagar PJ & Disney HJ (1975) The birds of Lord Howe Island: a report on the rare and endangered species. ICBP Bulletin 12, 187–202.
Garnett ST & Baker GB (2021) The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne.
Garnett ST & Crowley GM (2000) The Action Plan for Australian Birds. Environment Australia, Canberra.
Halpin LR, Mott R, Clay TA, Humphries GRW, Chatwin TA, Carlile N & Clarke RH (2022) Predicting the foraging habitats of sympatrically breeding gadfly petrels in the South Pacific Ocean. Frontiers in Marine Science 9, 853104.
Merton DV (1970) Kermadec Island expedition reports: a general account of bird life. Notornis 17, 147–199.
Schodde R, Fullagar P & Hermes N (1983) A review of Norfolk Island birds past and present (Special Publication No. 8). Australian National Parks and Wildlife Service, Canberra.
Pterodroma nigripennis—black-winged petrel
Conservation significance
EPBC Act Listing Status: Marine
Non-statutory Listing Status: Listed as Vulnerable under the Biodiversity Conservation Act 2016 (NSW).
Non-statutory Listing Status: Described as least concern in the Action Plan for Australian Birds 2010 (Garnett et al. 2011).
Distribution
The black-winged petrel (Pterodroma nigripennis) species has a wide oceanic range in the Tasman Sea and in the subtropical and tropical regions of the Central Pacific Ocean. It breeds on Lord Howe Island, the Norfolk Island Group, Kermadec Island Group, Three Kings Group, the Chatham Islands and the Austral Group. In the Norfolk Island Group, it is an uncommon breeding summer migrant that is present from late November to early March, primarily breeding on Phillip Island (Schodde et al. 1983). The species has attempted to breed on Norfolk Island, but mortality has been high probably due to predation from feral cats and rats (Schodde et al. 1983). Between 1978-79, there were between 50 and 100 pairs breeding on Phillip Island (Tarburten 1981); in 1985, several hundred birds (Hermes et al. 1986); in 2006, 1000-10,000 pairs (Priddel et al. 2010); and in 2017–18, 18,000 pairs (Carlile & O’Dwyer 2018).
Ecology
A summer breeder. A single egg is laid and incubated for 45 days, with fledging occurring after an additional 85 days. Recent breeding success increased from 47% in 2017 to 64% in 2021, an increase likely due to the control of purple swamphen numbers on Phillip Island since 2019 (O’Dwyer et al. 2023). Excavates burrows under rocks or vegetation to nest, in upper valleys and shallow soil under cliff faces.
Foraging: feeds on small fish, squid and crustaceans, which are captured by surface seizing and dipping (Halpin et al. 2022). Not known to frequent at-sea areas of high plastic concentrations in breeding or non-breeding periods (Clarke et al. 2023).
Habitat
Migratory and highly pelagic; migrates to the Central Pacific when not breeding.
Threats
The main threats to the black-winged petrel are the reduction in the quality of foraging areas through climate related shifts in oceanic resources, and predation from rats and cats on Norfolk Island. On Phillip Island, other threats include predation of nests by purple swamphens and the degradation and loss of breeding habitat. The Phillip Island centipede (Cormocephalus coynei) is known to reduce fledgling numbers annually (Halpin et al. 2021) but is unlikely to be impacting recruitment into the population.
Impact on other species
None known.
Management actions
Continue control of swamphens on Phillip Island. Protect and enhance nesting areas through revegetation efforts on Phillip Island. Retain status of pest-free Phillip Island through detection monitoring for introduced vertebrates and invertebrates. Protect potential nesting sites on Norfolk Island from feral cats and rats to allow re-establishment of colonies there.
Relevant literature
Carlile N & O’Dwyer T (2018) NI2016–26 Report to the Director national parks and Manager Norfolk Island National Park. Office of Environment and Heritage NSW.
Carlile N, Halpin LR, O’Dwyer T, O’Neill L (2023) Changing fortunes of the Black-winged Petrel Pterodroma nigripennis following the Lord Howe Island Rodent Eradication Project - interactions with other recovering species. Bird Conservation International 33: e18.
Christian M (2005) Norfolk Island … the birds. Green Eyes Publications, Norfolk Island.
Clark BL, Carneiro APB, Pearmain EJ et al. (2023) Global assessment of marine plastic exposure risk for oceanic birds. Nature Communications 14, 3665.
Garnett ST, Szabo J & Dutson G (2011) The Action Plan for Australian Birds 2010. CSIRO Publishing, Melbourne.
Halpin LR, Mott R, Clay TA, Humphries GRW, Chatwin TA, Carlile N & Clarke RH (2022) Predicting the foraging habitats of sympatrically breeding gadfly petrels in the South Pacific Ocean. Frontiers in Marine Science 9, 853104.
Halpin LR, Terrington DI, Jones HP, Mott R, Wong WW, Dow DC, Carlile N & Clarke RH (2021) Arthropod predation of vertebrates structures trophic dynamics in island ecosystems. The American Naturalist 198(4), 540-550.
Hermes N, Evans O & Evans B (1986) Norfolk Island birds: a review 1985. Notornis 33, 141–149.
O’Dwyer T, Carlile N, O’Neill L & Halpin LR (2023) Changing fortunes of the Black-winged Petrel Pterodroma nigripennis following the Lord Howe Island Rodent Eradication Project - interactions with other recovering species. Bird Conservation International 33: e18.
Priddel D, Carlile N, Evans O, Evans B & McCoy H (2010) A review of the seabirds of Phillip Island in the Norfolk Island Group. Notornis 57, 113–127.
Schodde R, Fullagar P & Hermes N (1983) A review of Norfolk Island birds past and present (Special Publication No. 8). Australian National Parks and Wildlife Service, Canberra.
Tarburton MK (1981) Seabirds nesting on Norfolk Island. Notornis 28, 209–211.
Pterodroma solandri—providence petrel
Conservation significance
EPBC Act Listing Status: Marine.
State Listing Status: Listed as Vulnerable under the Biodiversity Conservation Act 2016 (NSW).
Non-statutory Listing Status: Described as least concern in the Action Plan for Australian Birds 2020 (Garnett & Baker 2021).
Distribution
The providence petrel (Pterodroma solandri) was discovered on Norfolk Island in 1788 and was considered common, with large breeding colonies on Mt Pitt and Mt Bates. The birds were an important source of food for the early settlers and more than 170,000 birds were harvested between April and July 1790 (Medway 2002). By 1796 the population had dropped to about 15,000 and by 1800 the species was extirpated from Norfolk Island (Lindsey 1986).
The providence petrel returned over 150 years later to Phillip Island in 1985 (Hermes et al. 1986). Genetic studies on the providence petrel have shown that there is high gene flow between the populations on Lord Howe and Phillip Islands, suggesting that the Phillip Island population is the result of recent colonisation from Lord Howe, rather than a relict population from the extinct Norfolk Island population (Davidson 2008; Carlile 2011; Lombal et al. 2016).
In May 2011, 35 birds were counted in the air at one time and 252 extant burrows documented along ridge-lines and above Cow Bay on Phillip Island (Carlile 2011). In the early 2000s, 32,000 breeding pairs were estimated to be present on Lord Howe Island (Bester 2003), which remains the principal location of breeding for this species. Feral cats have likely prevented this species re-establishing on Norfolk Island.
Ecology
Breeding occurs on Phillip Island from February to November, with egg-laying occurring in May. Adults share incubation for 55 days and feeding of young over the following three months. Nests in a chamber at the end of a burrow.
Diet consists of squid, fish and crustaceans. While feeding chicks, adults make foraging trips of 1 to 14 days and return to feed chicks in the late afternoon and through the night.
Habitat
Marine, pelagic in waters 15-25 C. Breeds on the upper slopes along and below ridgelines on Phillip Island.
Threats
The main threats to the providence petrel are the reduction in the quality of foraging areas through climate-related shifts in oceanic resources, and some competition with wedge-tailed shearwaters (Ardenna pacifica) at restricted burrow sites where both species are present. Erosion in exposed areas continue to degrade some breeding areas, leading to a loss of habitat (Priddel et al. 2010). Offshore windfarms along the east coast of Australia may represent an emerging threat due to turbine strike.
Impact on other species
None known.
Risk assessment
The risk assessment is shown in Table 61 and Table 62.
Table 61 Risk assessment for Pterodroma solandri (current range, Phillip Island)
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Unlikely (11–25%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Major | Extreme |
4. Degradation of native vegetation through current or future grazing | Unlikely (11–25%) | Negligible | Negligible |
5. Lack of available nest sites | Unlikely (11–25%) | Minor | Low |
6. Predation by rodents | Possible (26–50%) | Minor | Low |
7. Predation by cats | Unlikely (11–25%) | Major | Medium |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Unlikely (11–25%) | Negligible | Negligible |
10. Predation by Argentine ant | Likely (51–90%) | Major | High |
11. Competition from/change of habitat because of weed invasion | Unlikely (11–25%) | Minor | Low |
12. Infection by pathogens already present | Unlikely (11–25%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Possible (26–50%) | Unknown | Unknown |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Likely (51–90%) | Major | High |
15. Problems caused by small populations, including lack of genetic diversity | Unlikely (11–25%) | Negligible | Negligible |
Table 62 Risk assessment for Pterodroma solandri (if range expanded to include Norfolk Island)
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Possible (26–50%) | Major | High |
2. Loss and fragmentation of native vegetation through current or future land clearing | Unlikely (11–25%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Possible (26–50%) | Moderate | Medium |
4. Degradation of native vegetation through current or future grazing | Unlikely (11–25%) | Minor | Low |
5. Lack of available nest sites | Unlikely (11–25%) | Negligible | Negligible |
6. Predation by rodents | Possible (26–50%) | Minor | Low |
7. Predation by cats | Almost certain (91–100%) | Extreme | Extreme |
8. Predation or damage by chickens | Rare (0–10%) | Minor | Negligible |
9. Predation by swamphens | Unlikely (11–25%) | Negligible | Negligible |
10. Predation by Argentine ant | Likely (51–90%) | Major | High |
11. Competition from/change of habitat because of weed invasion | Unlikely (11–25%) | Negligible | Negligible |
12. Infection by pathogens already present | Unlikely (11–25%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Possible (26–50%) | Unknown | Unknown |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Likely (51–90%) | Major | High |
15. Problems caused by small populations, including lack of genetic diversity | Unlikely (11–25%) | Minor | Low |
Management actions
Protect and enhance nesting areas through revegetation efforts on Phillip Island. Retain status of pest-free Phillip Island through detection monitoring for introduced vertebrates and invertebrates. As Phillip Island represents a significant second world population, regular monitoring is required to detect significant changes. Every three years, a survey of breeding burrows on Phillip Island should be undertaken in late May. From this, a subset of individual burrows (≥ 50) that are observable via visual inspection or using a burrow-scope and that contain a bird on an egg should be identified. These selected sites should be resurveyed in early October for the presence of a fledgling and to determine breeding success. The presence or absence of active wedge-tailed shearwater burrows nearby should be noted at this time to provide a possible indication of pre-fledging losses caused by intraspecific competition for nesting sites. Assess the potential for management of cat numbers within national park boundaries on Norfolk Island to make feasible the re-establishment of a colony on Mt Bates.
Relevant literature
Bester A (2003) The breeding, foraging ecology and conservation of the Providence Petrel Pterodroma solandri on Lord Howe Island, Australia. PhD Thesis, Charles Sturt University, Albury, NSW.
Binder D, Priddel D, Carlile N, & Kingsford RT (2013) Emergence, growth, ageing and provisioning of Providence Petrel (Pterodroma solandri) chicks: implications for translocation. Emu 113: 33–44.
Carlile N (2011) Observations of seabirds on Phillip Island 8–12 May 2011. Unpublished report.
Christian M (2005) Norfolk Island … the birds. Green Eyes Publications, Norfolk Island.
Davidson P (2008) Collection of Blood Samples from Providence Petrel Pterodroma solandri on Phillip Island, Norfolk Island Group 12–14 June 2008. Unpublished report.
Garnett ST & Baker GB (2021) The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne.
Hermes N, Evans O & Evans B (1986) Norfolk Island birds: a review 1985. Notornis 33, 141–149.
Lindsey TR (1986) The Seabirds of Australia: the national photographic index of Australian Wildlife. Angus and Robertson, North Ryde, NSW.
Lombal AJ, Wenner TJ, Carlile N, Austin JJ, Woehler E, Priddel D & Burridge CP (2016) Population genetic and behavioral variation of the two remaining colonies of Providence petrel (Pterodroma solandri). Conservation Genetics 18, 117–129.
Medway DG (2002) History and causes of the extirpation of the Providence petrel (Pterodrma solandri) on Norfolk Island. Notornis 49 (4), 246–258.
Priddel D & Carlile N (2007) Conservation and Restoration of seabird populations within the Norfolk Island Group. NSW Department of Environment and Conservation. Unpublished report.
Priddel D, Carlile N, Evans O, Evans B & McCoy H (2010) A review of the seabirds of Phillip Island in the Norfolk Island Group. Notornis 57, 113–127.
Puffinus assimilis—little shearwater
Conservation significance
EPBC Act Listing Status: Marine.
State Listing Status: Listed as Vulnerable under the Biodiversity Conservation Act 2016 (NSW).
Non-statutory Listing Status: Described as Least Concern in the Action Plan for Australian Birds 2020 (Garnett & Baker 2021).
Distribution
The little shearwater (Puffinus assimilis) is a widespread species of the subtropical Atlantic, Indian and Pacific Oceans. Breeds on islands of the Lord Howe Group, the Kermadec Islands, the Norfolk Group, and islands off the West Australian coast.
Withing the Norfolk Island Group, the little shearwater breeds on Phillip and Nepean Islands and has been reported from Anson Point in the mid-1970s. Predation by feral cats and rats has apparently eliminated colonies from the main island. On Phillip Island, Priddel et al. (2010) estimated the population to be 100–1000 pairs.
Ecology
A winter breeder present in the Norfolk Island Group from April (Priddel et al. 2010) and breeding between July and early December (Schodde et al. 1983). Nests in a narrow burrow. A single egg is laid and incubated for 55 days, with fledging occurring after an additional 72 days.
Diet consists of small fish, squid and krill, which is captured by surface diving, pursuit plunging, and by surface seizing.
Habitat
Marine, pelagic, breeds on subtropical and subantarctic islands; digs burrows in soft soil under mats of succulents, in grassland, under low shrubs, among loose rocks in upper valleys, and shallow soil under cliff faces.
Threats
The main threats to the little shearwater are the reduction in the quality of foraging areas through climate-related shifts in oceanic resources, and predation from rats, cats and kestrels on Norfolk Island. On Phillip Island, additional threats include predation of nests by purple swamphens and degradation and loss of breeding habitat.
Impact on other species
None known.
Management actions
Continue control of swamphens on Phillip Island. Protect and enhance nesting areas through revegetation efforts on Phillip Island. Retain status of pest-free Phillip and Nepean islands through detection monitoring for introduced vertebrates and invertebrates.
Relevant literature
Garnett ST & Baker GB (2021) The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne.
Priddel D, Carlile N, Evans O, Evans B & McCoy H (2010) A review of the seabirds of Phillip Island in the Norfolk Island Group. Notornis 57, 113–127.
Schodde R, Fullagar P & Hermes N (1983) A review of Norfolk Island birds past and present (Special Publication No. 8). Australian National Parks and Wildlife Service, Canberra.
Sula dactylatra—masked booby
Conservation significance
EPBC Act Listing Status: Marine, migratory.
State Listing Status: Listed as Vulnerable under the Biodiversity Conservation Act 2016 (NSW).
Non-statutory Listing Status: Described as Least Concern in the Action Plan for Australian Birds 2020 (Garnett & Baker 2021).
Distribution
The masked booby (Sula dactylatra) is widely distributed throughout the tropical and subtropical seas of the world. In Australia it breeds on islands off north-east and north-west Australia.
In the Norfolk Island Group, the species nests regularly on Nepean and Phillip islands. Boobies have attempted to nest on rocky islets off the coast and began establishing a colony at Rocky Point on the main island, which persisted for a few years before some birds were killed and the colony dispersed. Long-term banding of this species has suggested a marked decline (Christian 2005). Coyne et al. (2015) estimated substantial variation in the number of pairs on Nepean Island each year between 1978 to 1995 (ranging from 140 pairs to 1090 pairs). Numbers banded between 1978–1984 were higher than between 1996–2007, suggesting a decline in the breeding population size over that period (Coyne et al. 2015). In 2006 the population on Phillip Island was estimated to exceeded 300 breeding pairs, based on previous years of fledgling banding (Priddel et al. 2010).
In recent years, a growing population has formed on the northern cliffs of Fisherman’s Lane (on Norfolk Island) as a result of targeted and coordinated pest animal control by private landholders.
Ecology
Has a protracted breeding season from August to February with the main egg-laying period occurring in October. Incubation is 45 days, and the period from hatching to fledging spans 120 days. Out of a clutch of two eggs only one fledgling is raised. Lost eggs will be replaced.Nests on the ground in high open areas.
Diet consists of squid and fish.
Habitat
Marine, pelagic mainly in tropical and subtropical waters. Breeds on high open areas so it can take off into the wind.
Threats
The main threat to the masked booby is reduction in the quality of foraging areas through climate‑related shifts in oceanic resources. Predation from cats and disturbance from humans, rats and dogs limit breeding on Norfolk Island.
Impact on other species
None known.
Management actions
Protect nesting areas. Every three years, carry out a census of breeding pairs (by drone counts in early November) to detect significant changes. Retain status of pest-free Phillip Island through detection monitoring for introduced vertebrates and invertebrates.
Relevant literature
Christian M (2005) Norfolk Island … the birds. Green Eyes Publications, Norfolk Island.
Coyne P, Evans B, Evans O & McCoy H (2015) The Tasman Masked Booby Sula dactylatra tasmani of Nepean and Phillip Islands in the Norfolk Island Group. Corella 39 (3), 60–66.
Garnett ST & Baker GB (2021) The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne.
Priddel D, Carlile N, Evans O, Evans B & McCoy H (2010) A review of the seabirds of Phillip Island in the Norfolk Island Group. Notornis 57, 113–127.
Schodde R, Fullagar P & Hermes N (1983) A review of Norfolk Island birds past and present (Special Publication No. 8). Australian National Parks and Wildlife Service, Canberra.
Tarburton MK (1981) Seabirds nesting on Norfolk Island. Notornis 28, 209–211.
Abutilon julianae—Norfolk Island abutilon
Family MALVACEAE
Conservation significance
Endemic to Norfolk Island Group
EPBC Act Listing Status: Critically Endangered
Description
A subshrub to about 1m tall with young stems covered with dense stellate hairs. Leaves with petiole 2–8 cm long; the blade of the leaf is heart shaped, hairy on the underside and almost hairless on top; solitary yellow flowers.
Distribution and abundance
Originally occurred on Norfolk Island and on Phillip Island but was lost from Norfolk Island. Rediscovered on Phillip Island after the eradication of rabbits in the 1980s (Mills 2012b). By 1988, there were about 100 small-to-medium plants and 12 medium-to-large plants known, mostly occurring over the inaccessible southern part of Phillip Island. There were three main patches: one of about 100 plants, another with 18 plants and one with about 10 plants (Sykes & Atkinson 1988).
Mills (2009b) counted 43 plants on Phillip Island (in cliff edge shrubland and pigface herbland) including mature plants and seedlings, but suggested the population was greater than this figure implies.
Abutilon julianae has now been extensively planted on Norfolk Island in the national park in open areas, and the population is increasing with increased management intervention and use of the species in rehabilitation works. The population estimate in 2021 was 227 individuals. Propagation and planting have occurred through the Norfolk Island National Park threatened flora program.
The distribution is shown in Map 23.
Ecology
Little known.
Habitat
Grows in open situations among grasses; probably previously restricted to exposed coastal sites.
Threats
Major current threats include weed invasion and competition, as well as predation by chickens. Phytophthora cinnamomi is potentially a major risk.
Map 23 Distribution of Abutilon julianae
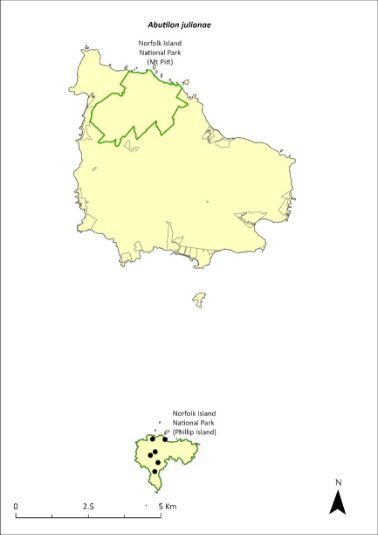
Green outlines indicate reserves within which the species can be found. Points show recorded locations (Mills 2009b).
Impact on other species
None known.
Risk assessment
Risk assessment undertaken for Critically Endangered trees/shrubs as a grouping. The risk assessment is shown in Table 63.
Table 63 Risk assessment for Critically Endangered trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Possible (26–50%) | Minor | Low |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Likely (51–90%) | Moderate | Medium |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Major | High |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Moderate | High |
Management actions
Continue re-establishing a wild population on Norfolk Island by raising plants from seeds and planting out established plants in suitable habitat with protection from invasive herbivores; undertake regular monitoring to identify factors affecting their survival. Implement targeted weed control and maintenance and ongoing rat and chicken control. Exclude grazing from areas known to contain A. julianae.
Recovery target
The recovery target is shown in Table 64.
Table 64 Recovery target for Abutilon julianae
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Critically Endangered | 227 | >95% within the national park | 1000 |
Relevant literature
Mills K (2009b) The Vegetation of Phillip Island, Norfolk Island Group. Envirofund 2007/2008. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
Achyranthes arborescens—chaff tree, soft-wood
Family AMARANTHACEAE
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Critically Endangered.
Description
Soft-wooded trees to 9m tall.
Distribution and abundance
Occurs in in viny hardwood forest, in valleys extending southwards and south-east from Mt Pitt and Mt Bates, with 99% of the natural population in Norfolk Island National Park (Sykes & Atkinson 1988).
Only 55 individuals were located in 1988 (Sykes & Atkinson 1988) and the species had declined further by 1989, particularly in Filmy Fern Gully (Gilmour & Helman 1989b).
The total number of mature individuals in 2003 was 57 and the population was severely fragmented, with none of the four subpopulations containing more than 40 individuals (TSSC 2003d). Outside the national park it occurs in moist valleys, with three mature trees and some natural regeneration identified.
Propagation and planting in the national park, particularly in the valleys, greatly increased the population of the species. 109 plants were recorded in 2012 ranging from seedlings to trees, and mainly growing on valley floors where the species had been planted (Mills 2012b). A few individuals have also been planted in Hundred Acres Reserve and Selwyn Reserve.
The population estimate in 2021 was 391 individuals. Propagation has occurred through the Norfolk Island National Park threatened flora program.
The distribution is shown in Map 24.
Ecology
Requires canopy gaps to successfully establish. Established seedlings on valley floors can be washed away by heavy rain.
Habitat
Achyranthes arborescens can grow in the shade of Norfolk palm (Rhopalostylis baueri), occasionally on ridges but most commonly in gullies on valley floors or lower valley sides.
Threats
Weed invasion and competition, especially in suitable canopy gaps that are filled rapidly by wild tobacco (Solanum mauritianum) and vines such as coastal morning glory (Ipomoea cairica), which smothers young and adult plants (Sykes & Atkinson 1988). A. arborescens is also threatened by cattle grazing and predation of seeds by rats. Phytophthora cinnamomi is potentially a major risk.
Map 24 Distribution of Achyranthes arborescens
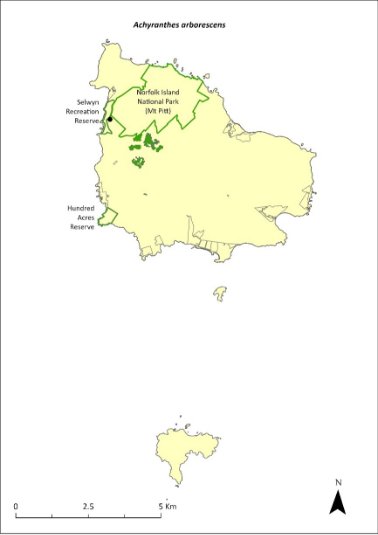
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021). Points show recorded locations (Mills 2017d).
Impact on other species
None known.
Risk assessment
Risk assessment undertaken for Critically Endangered trees/shrubs as a grouping. The risk assessment is shown in Table 65.
Table 65 Risk assessment for Critically Endangered trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Likely (51–90%) | Major | High |
4. Degradation of native vegetation through current or future grazing | Rare (0–10%) | Minor | Negligible |
6. Predation by rodents | Likely (51–90%) | Moderate | Medium |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Likely (51–90%) | Moderate | Medium |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Major | High |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Possible (26–50%) | Major | High |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Almost certain (91–100%) | Major | Extreme |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Major | Extreme |
Management actions
Continue propagation and planting within canopy breaks following weed control. The species is difficult to propagate from seed but propagates well from cuttings. Remove introduced species such as wild tobacco and coastal morning glory in proximity to established plants to promote natural regeneration (Sykes & Atkinson 1988). Manage or exclude grazing in areas know to contain A. arborescens. Carry out rodent and chicken control.
Recovery target
The recovery target is shown in Table 66.
Table 66 Recovery target for Achyranthes arborescens
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Critically Endangered | 391 | >95% within the national park 1% in the reserves | 1000 |
Relevant literature
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
Gilmour PM & Helman CE (1989b) The Vegetation of Norfolk Island National Park. Report to the Australian National Parks and Wildlife Service, Norfolk Island.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2017d) Survey of public reserves on Norfolk Island for threatened plant species: 6. Anson Bay Reserve and Selwyn Reserve. Prepared for Norfolk Island Regional Council.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003d) Commonwealth Listing Advice—Critically Endangered Achyranthes arborescens (Chaff Tree, Soft-wood).
Achyranthes margaretarum—Phillip Island chaffy tree
Family AMARANTHACEAE
Conservation significance
Endemic to Phillip Island.
EPBC Act Listing Status: Critically Endangered.
Description
A compact shrub to 2–3 m high with maroon flowers.
Distribution and abundance
A single 2 m tall Achyranthes margaretarum specimen was discovered on Phillip Island in the late 1980s following the removal of rabbits. Although this specimen died in the early 1990s it left numerous seedlings, of which ten or so survived to maturity (de Lange & Murray 2001). By March 1999 the wild population stood at 10 adult specimens together with numerous saplings and seedlings. These wild occurrences were further supplemented by the successful translocation of 10 additional seedlings to other parts of the island (de Lange & Murray 2001).
Mills (2009b) noted that plants were naturally regenerating on Phillip Island, but the population was quite small; 22 plants were found, ranging in size from seedlings to shrubs, seven of which were over one metre tall. It is now found in the upper section of Long Valley at Owen’s Camp on Phillip Island (Mills 2009b; M Wilson 2024. pers comm 12 January). Counts in 2021 showed a decline in the population to 14.
A. margaretarum has been established back on Norfolk Island in revegetation plantings within the national park.
The distribution is shown in Map 25.
Ecology
Reaches sexual maturity within two years from seed germination (de Lange & Murray 2001).
Habitat
Found growing at 180 m above sea level, in association with Norfolk Island flax (Phormium tenax), under a dense canopy of white oak (Lagunaria patersonia). The plants show a preference for canopy gaps where flax growth is less dense (de Lange & Murray 2001).
Threats
Prior to feral animal eradication on Phillip Island, A. margaretarum was threatened by grazing from pigs, goats and rabbits. The loss of vegetation and high levels of erosion from that grazing now reduces the ability of the species to recolonise the island. The weed species bleeding heart (Homolanthus populifolius) and wild tobacco (Solanum mauritianum) compete with A. margaretarum and may threaten its establishment in high-light areas. The species is also threatened by small population size, which can lead to low genetic diversity and an increased risk of extinction through natural events such as cyclones, slips and drought. Phytophthora cinnamomi is potentially a major risk.
Map 25 Distribution of Achyranthes margaretarum
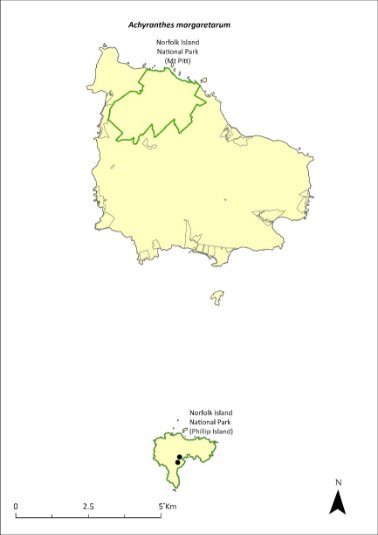
Green outlines indicate reserves within which the species occurs. Points show recorded locations (Mills 2009b).
Impact on other species
None known.
Risk assessment
Risk assessment undertaken for Critically Endangered trees/shrubs as a grouping. The risk assessment is shown in Table 67.
Table 67 Risk assessment for Critically Endangered trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Major | Extreme |
4. Degradation of native vegetation through current or future grazing | Rare (0–10%) | Minor | Negligible |
6. Predation by rodents | Rare (0–10%) | Unknown | Unknown |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Likely (51–90%) | Moderate | Medium |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Major | High |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Possible (26–50%) | Moderate | Medium |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Almost certain (91–100%) | Major | Extreme |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Extreme | Extreme |
Management actions
Undertake propagation in the Phillip Island nursery and continue planting at Phillip Island (though plantings on Norfolk Island may act as an insurance population). Continue habitat restoration, including targeted weed control, at Phillip Island to provide suitable habitat for the species.
Recovery target
The recovery target is shown in Table 68.
Table 68 Recovery target for Achyranthes margaretarum
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Critically Endangered | 14 | 100% within the national park | 500 |
Relevant literature
de Lange PJ & Murray BG (2001) A new Achyranthes (Amaranthaceae) from Phillip Island, Norfolk Island Group, South Pacific Ocean. New Zealand Journal of Botany 39, 1–8.
Mills K (2009b) The Vegetation of Phillip Island, Norfolk Island Group. Envirofund 2007/2008. Kevin Mills & Associates, Jamberoo, NSW.
Wilson M (2024) Personal communication by email, 12 January 2024, Parks Australia (Norfolk Island National Park).
Anthosachne kingiana kingiana—Phillip Island wheat-grass
Family POACEAE
Conservation significance
Endemic to Norfolk Island and Lord Howe Island.
EPBC Act Listing Status: Critically Endangered.
For further information on the species outside of the Norfolk Island Group, see the species profile on SPRAT.
Description
A tufted perennial grass growing 30–100 cm tall with glaucous leaves.
This species is cryptic unless containing flowering heads and difficult to find. It is easily confused with Elymus spp.
Distribution and abundance
A very rare grass found only on Phillip Island, Anthosachne kingiana kingiana was rediscovered in 1987 following the removal of rabbits. Several small patches were found growing on north facing slopes towards the upper part of the island (Sykes & Atkinson 1988).
A. kingiana kingiana has not been recorded on Norfolk Island since 1963 (DEH 2003). It was previously reported from Second Sands and Point Ross (Connor 1990; Mills 2009b). Occurrences of the species on Norfolk Island are likely to be through artificial propagation and translocation (DEH 2003).
There were fewer than 50 mature individuals in 2003 (TSSC 2003a). Two small populations were found by Mills (2009b) above the dykes area and on the cliff edge on the southern side of Stony Valley on Phillip Island. The estimated population in 2021 was five individuals.
The distribution within the Norfolk Island Group is shown in Map 26.
Ecology
Little known.
Habitat
Grows on the north-facing slopes of Phillip Island in high areas, among tall herbs and subshrubs, often in association with a herb community dominated by pigface (Carpobrotus glaucescens).
Threats
Prior to feral animal eradication on Phillip Island, the major threat to the species was grazing from pigs, goats and rabbits. The loss of vegetation and high levels of erosion from that grazing now reduces the ability of the species to recolonise the island.
Current threats are the small population size and restricted distribution (Phillip Island) and as a result, an increased risk of extinction through natural events such as cyclones, slips and drought. Weed invasion and competition is also a threat. Grazing by herbivores may be a threat if propagated on Norfolk Island.
Map 26 Distribution of Anthosachne kingiana kingiana
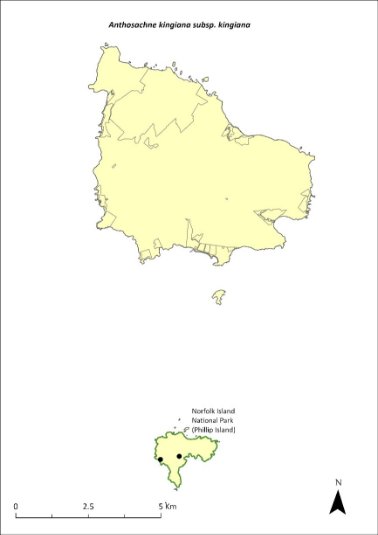
Green outlines indicate reserves within which the species occurs. Points show recorded locations (Mills 2009b).
Impact on other species
Little known.
Risk assessment
Risk assessment undertaken for Critically Endangered herbs/grasses as a grouping. The risk assessment is shown in Table 69.
Table 69 Risk assessment for Critically Endangered herbs/grasses as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Rare (0–10%) | Negligible | Negligible |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Almost certain (91–100%) | Major | Extreme |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Major | Extreme |
Management actions
Undertake surveys to search for individuals and conduct research to better understand the ecology of the species. Map distribution and determine conditions for successful germination. Monitor populations during late spring. Propagate on Phillip Island for use in rehabilitation work. Propagate on Norfolk Island and use to establish further populations in coastal habitat. Exclude grazing from any new populations established on Norfolk Island. Implement targeted weed control and maintenance.
The Lord Howe Island Biodiversity Management Plan covers the recovery needs of this species on the Lord Howe Island group. Possible future actions may need to be undertaken in collaboration with the NSW Government as appropriate.
Recovery target
The recovery target is shown in Table 70.
Table 70 Recovery target for Anthosachne kingiana kingiana
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Critically Endangered | 5 groups of plants | 100% within the national park | 100 groups of plants |
Relevant literature
Connor HE (1990) Elymus (Gramineae) on Norfolk Island. Kew Bulletin 45, 680.
Department of the Environment and Heritage (DEH) (2003) What the Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act) means for Norfolk Island. Department of the Environment and Heritage, Canberra.
Mills K (2009b) The Vegetation of Phillip Island, Norfolk Island Group. Envirofund 2007/2008. Kevin Mills & Associates, Jamberoo, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003a) Commonwealth Listing Advice for Norfolk Island Flora – 11 Critically Endangered Species.
Blechnum norfolkianum—Norfolk Island water-fern
Family BLECHNACEAE
Conservation significance
Found on Norfolk Island and on other Pacific Islands.
EPBC Act Listing Status: Endangered.
Description
A medium-sized terrestrial fern with fronds growing to between 30 and 80cm long and a short erect rhizome (underground stem).
Distribution and abundance
Australian distribution is restricted to Norfolk Island but also occurs in the Kermadec Islands, Vanuatu, Samoa and the Society Islands (TSSC 2003c). It is listed in New Zealand as “at risk—sparse” because it exists as widely scattered, small subpopulations or has restricted ranges (de Lange et al. 2004).
The total number of plants recorded in 2012 in Norfolk Island National Park was 708, including small plants to mature individuals (Mills 2012b). Plants ranged from small ferns to plants over one metre in height, although most specimens were under 50 cm tall. There were marked differences between populations in the moister, southern valleys and the drier valleys on the northern side of the mountains. The number of plants counted in the wetter valleys averaged 22 plants per 100 metres of transect, while in the drier valleys the figure was 0.4 plants per 100 metres (Mills 2012b). The population is considered to have remained stable since 2012.
The distribution is shown in Map 27.
Ecology
The species is found in damp and shady places mostly occurring in the south-facing valleys of the Mt Pitt section of the national park (Sykes & Atkinson 1988), particularly on the upper slopes of Mt Bates.
Habitat
The species occupies moist palm valley forest along the watercourses in the national park, particularly those on the moister, southern side of the mountains (Mills 2012b).
Threats
Drought/dry conditions due to climate change. Changes to hydrology within the national park (Braggins 1996). Weed invasion and competition.
Impact on other species
None known.
Map 27 Distribution of Blechnum norfolkianum
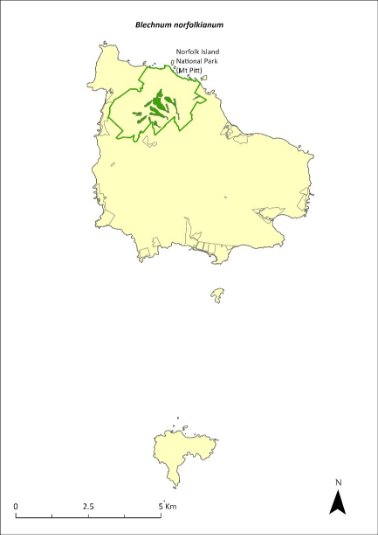
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021).
Risk assessment
Risk assessment undertaken for Endangered ferns as a grouping. The risk assessment is shown in Table 71.
Table 71 Risk assessment for Endangered ferns as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Rare (0–10%) | Negligible | Negligible |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Possible (26–50%) | Moderate | Medium |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Likely (51–90%) | Major | High |
15. Problems caused by small populations, including lack of genetic diversity | Possible (26–50%) | Major | High |
Management actions
Implement targeted weed control and maintenance. Implement revegetation/habitat restoration. Investigate potential for ex situ conservation (germplasm storage, propagation, replanting, representation in botanic gardens collections).
Recovery target
The recovery target is shown in Table 72.
Table 72 Recovery target for Blechnum norfolkianum
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Critically Endangered | 5 | 100% within the national park | 100 groups of plants |
Relevant literature
Braggins JE (1996) Report on the conservation status of the ferns of Norfolk Island. Unpublished report to the Australian Nature Conservation Agency.
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
de Lange PJ, Johnson PN, Norton DA, Hitchmough R, Heenan PB, Courteney SP, Molloy B.P.J, Ogle C.C & Rance BD (2004) Threatened and uncommon plants of New Zealand. New Zealand Journal of Botany 42, 45–76.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003c) Commonwealth Listing Advice for Norfolk Island Flora – 16 Endangered Species.
Boehmeria australis australis—tree nettle, nettletree
Family URTICACEAE
Conservation significance
Endemic to Norfolk Island
EPBC Act Listing Status: Critically Endangered
Description
A small tree or large spreading shrub to 5m tall; monoecious (male and female flowers separate but found on the same tree).
Distribution and abundance
Found in the valleys east of Mt Bates and Mt Pitt, with only 16 individuals sighted during surveys in 1988 (Sykes & Atkinson 1988). Later surveys suggested Boehmeria australis australis was less rare than indicated with small numbers recorded at several sites on the northern side of Mt Bates, and about 30 individuals found in a protected forest remnant on private land (Gilmour & Helman 1989a).
By 2003 there were 33 mature individuals (TSSC 2003a), with a few healthy trees occurring in the north-east corner of the national park. Mills (2012b) noted that regeneration from planted specimens and natural recruitment had increased the population of B. australis australis significantly since 2003 and found a total of 259 plants in the national park, ranging from seedlings to mature plants.
The population has since increased to 591 individuals through propagation and planting as part of the Norfolk Island National Park threatened flora program.
The distribution is shown in Map 28.
Ecology
B. australis australis is a rapidly growing species with a short life span that is adapted to colonising extensive open sites where the ground has been exposed.
Habitat
Grows in an open sheltered habitat on the margins of rainforest remnants (Sykes & Atkinson 1988, Gilmour & Helman 1989a).
Threats
Weed invasion and competition from weeds such as Lantana (Lantana camara), William Taylor (Ageratina riparia), kikuyu grass (Cenchrus clandestinus) and wild tobacco (Solanum mauritianum). The species is also threatened by grazing in areas outside of the national park and attack by phytophagous insects (Sykes & Atkinson 1988). Phytophthora cinnamomi is potentially a major risk.
Map 28 Distribution of Boehmeria australis australis

Green outlines indicate reserves within which the species occurs.
Impact on other species
None known.
Risk assessment
Risk assessment undertaken for Critically Endangered trees/shrubs as a grouping. The risk assessment is shown in Table 73.
Table 73 Risk assessment for Critically Endangered trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Likely (51–90%) | Minor | Medium |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Likely (51–90%) | Moderate | Medium |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Major | High |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Major | Extreme |
Management actions
Implement targeted weed control and maintenance to promote natural regeneration around mature plants of B. australis australis just before seed drop (summer and autumn; Sykes & Atkinson 1988). Establish populations through propagation and planting in suitable gully habitats in the public reserves; manage or exclude cattle grazing.
Recovery target
The recovery target is shown in Table 74.
Table 74 Recovery target for Boehmeria australis australis
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Critically Endangered | 591 | >95% within national park | 1000 |
Relevant literature
Gilmour PM & Helman CE (1989a) A Survey of Quality Plant Communities of Norfolk Island Outside the national park. Report to the Australian National Parks and Wildlife Service, Norfolk Island.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003a) Commonwealth Listing Advice for Norfolk Island Flora – 11 Critically Endangered Species.
Calystegia affinis—a creeper
Family CONVOLVULACEAE
Conservation significance
Found only on Norfolk Island and Lord Howe Island.
EPBC Act Listing Status: Critically Endangered
State Listing Status: listed as Critically Endangered under the Biodiversity Conservation Act 2016 (NSW)
For further information on the species outside of the Norfolk Island Group, see the species profile on SPRAT.
Description
A thin stemmed climbing or creeping vine with sparse leaves.
Distribution and abundance
On Norfolk Island, 95% of the natural population of Calystegia affinis is found in the open higher parts of Mt Pitt and Mt Bates, though it occasionally comes up from dormant seed when forest or scrub is cleared (Sykes & Atkinson 1988). In 2003 the Norfolk Island population consisted of about 45 mature individuals (TSSC 2003a). In 2012, 13 plants were counted across five transects (Mills 2012b). The plants were all on the higher parts of Mount Pitt and Mount Bates, except for one plant near the Red Stone Link Track. The population estimate in 2021 was 28 individuals.
C. affinis is also found on Lord Howe Island but it is very rare, known from only four locations with possibly only one plant at each location, sprawling over an area of some square metres.
The distribution within the Norfolk Island Group is shown in Map 29.
Ecology
Stems take root when touching the soil. The species grows prolifically in the sun under cultivation.
Habitat
Found on the open higher parts of mountain tops on Norfolk and Lord Howe Islands.
Threats
The primary threats to the species are weed invasion and competition, and habitat clearing and modification through track maintenance.
Impacts on Other Species
Can climb vigorously over other plants in cultivation.
Map 29 Distribution of Calystegia affinis
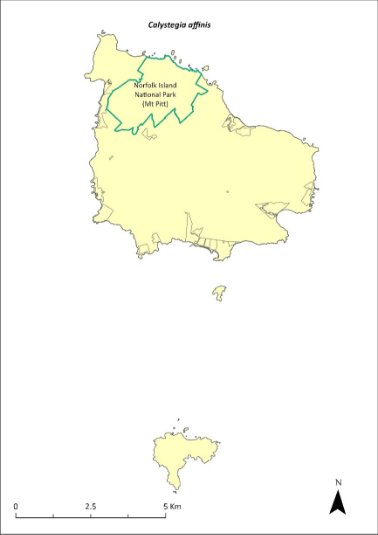
Green outlines indicate reserves within which the species occurs.
Risk assessment
Risk assessment undertaken for Critically Endangered vines/climbers as a grouping. The risk assessment is shown in Table 75.
Table 75 Risk assessment for Critically Endangered vines/climbers as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Almost certain (91–100%) | Major | Extreme |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Almost certain (91–100%) | Major | Extreme |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Extreme | Extreme |
Management actions
Undertake propagation and planting within suitable shaded areas. Undertake targeted weed control and maintenance.
The Lord Howe Island Biodiversity Management Plan covers the recovery needs of this species on the Lord Howe Island group. Possible future actions may need to be undertaken in collaboration with the NSW Government as appropriate.
Recovery target
The recovery target is shown in Table 76.
Table 76 Recovery target for Calystegia affinis
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Critically Endangered | 28 groups of plants | 100% in the national park | 100 groups of plants |
Relevant literature
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003a) Commonwealth Listing Advice for Norfolk Island Flora – 11 Critically Endangered Species.
Clematis dubia—clematis
Family RANUNCULACEAE
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Critically Endangered.
Description
A woody vigorous climber with hairy white flowers.
Distribution and abundance
Sykes and Atkinson (1988) note this vine was once common in the Mt Pitt and Mt Bates area but was not seen during surveys that year. In 2003 the natural population was confined to Norfolk Island National Park where there were 15 mature individuals recorded (TSSC 2003a).
Mills (2012b) found 53 plants but recorded only 3 as large plants. Seedlings were regularly found along transects. The species was also found on private land.
The population estimate has since increased to 303 individuals in 2021 through propagation and planting as part of the Norfolk Island National Park threatened flora program.
The distribution is shown in Map 30.
Ecology
This species will grow in light gaps and seeds rarely (every 4 to 5 years).
Habitat
This species grows on the forest margins and in clearings.
Threats
Weed invasion and competition.
Impact on other species
None known.
Map 30 Distribution of Clematis dubia
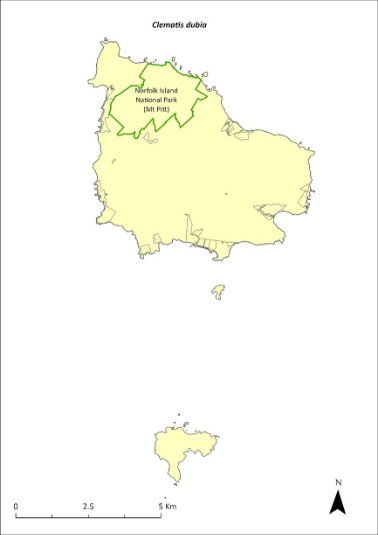
Green outlines indicate reserves within which the species occurs.
Risk assessment
Risk assessment undertaken for Critically Endangered vines/climbers as a grouping. The risk assessment is shown in Table 77.
Table 77 Risk assessment for Critically Endangered vines/climbers as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Unlikely (11–25%) | Major | Medium |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Moderate | Medium |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Extreme | Extreme |
Management actions
Continue propagation (from seeds and cuttings) and plantings within suitable areas. Undertake targeted weed control and maintenance around known plants. Undertake revegetation/habitat restoration.
Recovery target
The recovery target is shown in Table 78.
Table 78 Recovery target for Clematis dubia
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Critically Endangered | 303 | 100% within the national park | 500 |
Relevant literature
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003a) Commonwealth Listing Advice for Norfolk Island Flora – 11 Critically Endangered Species.
Coprosma baueri—coastal coprosma
Family RUBIACEAE
Conservation significance
Endemic to Norfolk Island and Phillip Island.
EPBC Act Listing Status: Endangered.
Description
A shrub or small tree with light green glossy leaves, small green flowers and orange egg-shaped fruit.
Distribution and abundance
Historical records suggest Coprosoma baueri may have always been rare (Gilmour & Helman 1989b). It occurs along the sea cliffs and slopes of Norfolk Island and on the higher parts of Phillip Island in cliff edge shrubland (Mills 2009b).
There were 228 mature individuals present in 2003 (TSSC 2003c).
In 2008 Mills (2009b) recorded 446 plants on Phillip Island. Surveys of the reserves in 2017 recorded five plants in Headstone Reserve, seven plants in Kingston Common, two plants in Two Chimneys Reserve, and 31 in Selwyn Reserve (Mills 2017c, d and g).
The population had increased to 708 individuals in 2021. Propagation and planting have occurred through the Norfolk Island National Park threatened flora program.
The distribution is shown in Map 31.
Ecology
Dioecious with wind-pollinated flowers and bird-dispersed seeds. The species can grow in areas affected by salt spray.
Habitat
Grows within coastal pine and white oak forest, coastal white oak shrubland, and coastal flax communities (Invasive Species Council & TierraMar 2021), as well as on cliffs and other locations on the coast. The species can be extensively chewed by insects. On Phillip Island, it grows mainly on the coastal cliffs, and the healthiest plants exist in areas of loose soil fertilised by wedge-tailed shearwaters (Ardenna pacifica) nesting on the cliffs (Sykes & Atkinson 1988).
Threats
C. baueri is threatened by drought on Phillip Island and by its small population size, which leads to an increased risk of extinction through natural events such as cyclones, slips and drought. Weed invasion and competition is also a threat. Possible hybridisation could occur with the introduced species C. repens (Sykes & Atkinson 1988). Phytophthora cinnamomi is potentially a major risk.
Map 31 Distribution of Coprosma baueri
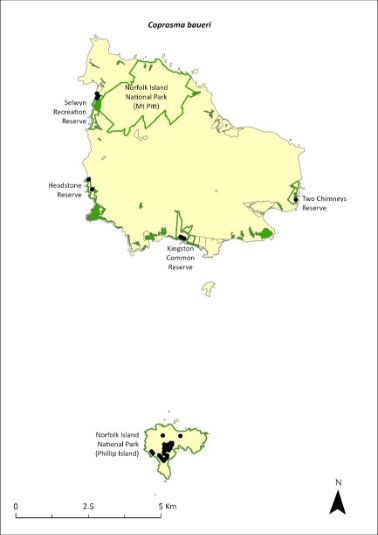
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021). Points show recorded locations (Mills2009b and Mills 2017c d and g).
Impact on other species
None known.
Risk assessment
Risk assessment undertaken for Endangered trees/shrubs as a grouping. The risk assessment is shown in Table 79.
Table 79 Risk assessment for Endangered trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Almost certain (91–100%) | Moderate | High |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Likely (51–90%) | Moderate | Medium |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Possible (26–50%) | Moderate | Medium |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Likely (51–90%) | Major | High |
Management actions
Continue propagation and planting within suitable areas. Undertake targeted weed control and maintenance around existing plants. Undertake revegetation/habitat restoration. Support conservation of wedge-tailed shearwaters to promote C. baueri regeneration, as these birds help to maintain a nutrient-rich open habitat through their burrowing habit (Sykes & Atkinson 1988).
Recovery target
The recovery target is shown in Table 80.
Table 80 Recovery target for Coprosma baueri
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Endangered | 708 | >90% within the national park 6% occurs within the reserves | 1500 |
Relevant literature
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
Gilmour PM & Helman CE (1989b) The Vegetation of Norfolk Island National Park. Report to the Australian National Parks and Wildlife Service, Norfolk Island.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Mills K (2009b) The Vegetation of Phillip Island, Norfolk Island Group. Envirofund 2007/2008. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2017c) Survey of public reserves on Norfolk Island for threatened plant species: 1. The Kingston Reserves. Prepared for Norfolk Island Regional Council.
Mills K (2017d) Survey of public reserves on Norfolk Island for threatened plant species: 6. Anson Bay Reserve and Selwyn Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017g) Survey of public reserves on Norfolk Island for threatened plant species: 5. Two Chimneys Reserve. Prepared for Norfolk Island Regional Council.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003c) Commonwealth Listing Advice for Norfolk Island Flora – 16 Endangered Species.
Coprosma pilosa—mountain coprosma
Family RUBIACEAE
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Endangered.
Description
Shrub or small tree to 6m tall with small green flowers, dark green hairy leaves and cone shaped dark bluish-purple fruit.
Distribution and abundance
The entire population of Coprosoma pilosa is found within the higher sections of Norfolk Island National Park and began to regenerate naturally following removal of cattle from the national park.
There were 260 individuals in 1995 (Anderson & Cochrane 1995) and 187 mature individuals in 2003 (TSSC 2003c). Mills (2012b) recorded 338 plants (ranging from small seedlings to mature trees) in the higher parts of the mountains in the national park. The population estimate in 2021 was 420 individuals.
The distribution is shown in Map 32.
Ecology
This species only seeds occasionally. Dioecious, with wind-pollinated flowers and bird-dispersed seeds.
Habitat
Occurs within moist upland hardwood forest and pine-hardwood ridge forest (Invasive Species Council & TierraMar 2021) and is almost entirely restricted to the higher parts of the mountains in the national park. Can be found down to about 180 metres, with very few growing at lower altitudes (Mills 2012b).
Threats
C. pilosa is threatened by its small population size/limited distribution and subsequent increased risk of extinction through natural events such as cyclones, slips and drought. Weed invasion and competition is also a threat. The epiphytic mistletoe (Ileostylus micranthus) favours C. pilosa as its host, and heavy infestations can kill the host plant. Changes in the climate of the mountain tops may threaten the species. Phytophthora cinnamomi is potentially a major risk.
Impact on other species
None known.
Map 32 Distribution of Coprosma pilosa

Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021).
Risk assessment
Risk assessment undertaken for Endangered trees/shrubs as a grouping. The risk assessment is shown in Table 81.
Table 81 Risk assessment for Endangered trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Almost certain (91–100%) | Moderate | High |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Likely (51–90%) | Moderate | Medium |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Possible (26–50%) | Moderate | Medium |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Almost certain (91–100%) | Major | Extreme |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Major | Extreme |
Management actions
Continue targeted weed control and maintenance around existing plants. Undertake revegetation/habitat restoration. Continue to exclude grazing.
Recovery target
The recovery target is shown in Table 82.
Table 82 Recovery target for Coprosma pilosa
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Endangered | 420 | >95% within the national park | 1000 |
Relevant literature
Anderson JG & Cochrane K (1995) Assessment of Population Numbers of Norfolk’s Threatened Plants (Norfolk Island National Park). Report to the Australian Nature Conservation Agency, Norfolk Island.
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
TSSC (Threatened Species Scientific Committee) (2003c) Commonwealth Listing Advice for Norfolk Island Flora – 16 Endangered Species.
Cordyline obtecta—Ti
Family AGAVACEAE
Conservation significance
Found only on Norfolk Island and in New Zealand.
EPBC Act Listing Status: Vulnerable.
Description
An erect shrub or tree to 10m tall with an erect pyramidal flower spike about 30cm long, grey bark, and whitish or blue-purple fruit.
Distribution and abundance
There were 818 mature individuals on Norfolk Island in 2003 (TSSC 2003b), with 65% of the population occurring within the Norfolk Island National Park. The population greatly improved over the next decade (Director of National Parks 2010) and had increased to 1863 individuals in 2021.
This species propagates well and has been planted in several public areas. On Phillip Island, it has been planted in the upper part of Long Valley (Mills 2009b). Abundant regeneration may take place following woody weed removal in the Mt Pitt section of the national park. The species has been planted in most of the public reserves.
The distribution is shown in Map 33.
Ecology
Little known.
Habitat
Grows in moist upland hardwood forest, pine-hardwood ridge forest, and lowland valley hardwood forest (Invasive Species Council & TierraMar 2021).
Threats
The main threats to Cordyline obtecta are cattle grazing and weed invasion and competition. Phytophthora cinnamomi is potentially a major risk.
Impact on other species
None known.
Map 33 Distribution of Cordyline obtecta
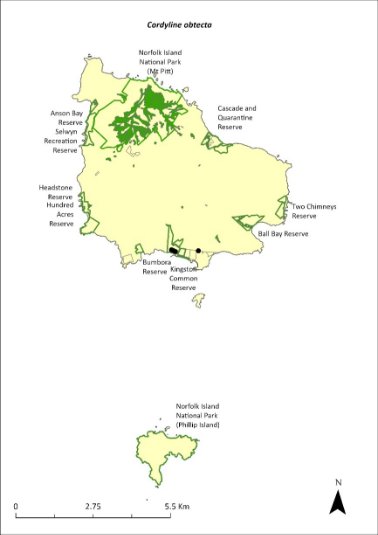
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021). Points show recorded locations (Mills2017c).
Risk assessment
Risk assessment undertaken for Vulnerable trees/shrubs as a grouping. The risk assessment is shown in Table 83.
Table 83 Risk assessment for Vulnerable trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Likely (51–90%) | Moderate | Medium |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Minor | Medium |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Unlikely (11–25%) | Minor | Low |
15. Problems caused by small populations, including lack of genetic diversity | Rare (0–10%) | Major | Low |
Management actions
Continue targeted weed control and maintenance. Undertake revegetation/habitat restoration. Continue replanting in public reserves. Exclude or manage cattle grazing in public reserves.
Recovery target
The recovery target is shown in Table 84.
Table 84 Recovery target for Cordyline obtecta
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Vulnerable | 1863 | >80% in the national park >15% in public reserves | 3000 |
Relevant literature
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Mills K (2009b) The Vegetation of Phillip Island, Norfolk Island Group. Envirofund 2007/2008. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2017c) Survey of public reserves on Norfolk Island for threatened plant species: 1. The Kingston Reserves. Prepared for Norfolk Island Regional Council.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
TSSC (Threatened Species Scientific Committee) (2003b) Commonwealth Listing Advice for Norfolk Island Flora - 15 Vulnerable Species.
Dendrobium brachypus—Norfolk Island orchid
Family ORCHIDACEAE
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Endangered.
Description
An epiphytic orchid with two or three pale cream flowers on a short stem.
Distribution and abundance
There were fewer than 200 mature individuals present on Norfolk Island in 2003 (TSSC 2003c).
More than 90% of the population is in the Mt Pitt section of the national park and in the botanic garden. The distribution is shown in Map 34.
Map 34 Distribution of Dendrobium brachypus

Green outlines indicate reserves within which the species occurs.
Ecology
Little known.
Habitat
Grows on tree branches in forest on the slopes of Mt Pitt.
Threats
Dendrobium brachypus is threatened by small population size and subsequent increased risk of extinction through natural events such as cyclones, slips and drought. Weed invasion and competition affecting host trees and climate change also threaten the species.
Impacts on other species
Grows on branches and stems of other plants but is not parasitic.
Risk assessment
Risk assessment undertaken for all threatened orchids as a grouping. The risk assessment is shown in Table 85.
Table 85 Risk assessment for all threatened orchids as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Minor | Medium |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Moderate | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Minor | Medium |
4. Degradation of native vegetation through current or future grazing | Unlikely (11–25%) | Moderate | Low |
6. Predation by rodents | Possible (26–50%) | Extreme | High |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Possible (26–50%) | Moderate | Medium |
12. Infection by pathogens already present | Unlikely (11–25%) | Moderate | Low |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Moderate | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Likely (51–90%) | Major | High |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Moderate | High |
Management actions
This orchid may require development of species-specific conservation actions, including ex situ conservation. Undertake targeted weed control and maintenance around host trees, and habitat protection and rehabilitation. Undertake research into the ecology of the species.
Monitor/survey likely areas of the national park after storms, rescue any fallen specimens and attempt to cultivate them in the Norfolk Island National Park Nursery (Sykes & Atkinson 1988).
Recovery target
The recovery target is shown in Table 86.
Table 86 Recovery target for Dendrobium brachypus
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Endangered | 200 | 100% in the national park | No decline |
Relevant literature
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003c) Commonwealth Listing Advice for Norfolk Island Flora – 16 Endangered Species.
Dysoxylum bijugum—sharkwood
Family MELIACEAE
Conservation significance
Australian distribution is restricted to Norfolk Island but also occurs in New Caledonia and Vanuatu (TSSC 2003b).
EPBC Act Listing Status: Vulnerable.
Description
A tree growing to 7m with yellow flowers and a strong fetid or garlic-like smell when bruised.
Distribution and abundance
The Norfolk Island population of Dysoxylum bijugum consisted of 870 mature individuals in 2003 (TSSC 2003b) with about 90% of the population occurring in the national park. It also occurs in the Mission Road rainforest fragments, at Steeles Point and in Anson Reserve, Selwyn Reserve and Two Chimneys Reserve. There are pockets in the south-east and west of the national park where this species is the dominant tree. In these areas there are many small seedlings. On Phillip Island, this species has been planted in the upper part of Long Valley (Mills 2009b). The 2021 population estimate was 940 individuals and is considered stable.
The distribution is shown in Map 35.
Habitat
Grows in moist upland hardwood forest, pine-hardwood ridge forest and plateau hardwood forest (Invasive Species Council & TierraMar 2021).
Ecology
Little known.
Threats
D. bijugum is threatened by cattle grazing and weed invasion and competition. Phytophthora cinnamomi is potentially a major risk.
Impact on other species
None known.
Map 35 Distribution of Dysoxylum bijugum

Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021).
Risk assessment
Risk assessment undertaken for Vulnerable trees/shrubs as a grouping. The risk assessment is shown in Table 87.
Table 87 Risk assessment for Vulnerable trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Likely (51–90%) | Moderate | Medium |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Possible (26–50%) | Minor | Low |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Minor | Low |
15. Problems caused by small populations, including lack of genetic diversity | Rare (0–10%) | Minor | Negligible |
Management actions
Undertake targeted weed control and maintenance. Undertake revegetation/habitat restoration. Exclude or manage cattle grazing. Undertake propagation and planting within suitable areas, including public reserves.
Recovery target
The recovery target is shown in Table 88.
Table 88 Recovery target for Dysoxylum bijugum
EPBC Act status | Estimated population (2023) | | 2034 target |
Vulnerable | 940 | >95% in the national park 1% in the reserves | 2000 |
Relevant literature
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Mills K (2009b) The Vegetation of Phillip Island, Norfolk Island Group. Envirofund 2007/2008. Kevin Mills & Associates, Jamberoo, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
TSSC (Threatened Species Scientific Committee) (2003b) Commonwealth Listing Advice for Norfolk Island Flora - 15 Vulnerable Species.
Elatostema montanum—mountain procris
Family URTICACEAE
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Critically Endangered.
Description
A fleshy, succulent-stemmed perennial herb or low shrub growing to 1m tall with a straggling habit.
Distribution and abundance
Gilmour & Helman (1989b) found the entire population of Elatostema montanum within the Mt Pitt section of the Norfolk Island National Park, and the largest known subpopulation declined between 1988 and 1989 (Gilmour & Helman 1989b).
Although not a detailed and comprehensive survey, 76 individuals were located in 2003 in seven severely fragmented subpopulations. No subpopulation contained more than 26 individuals, and only two subpopulations contained more than six individuals (TSSC 2003e).
In a 2012 survey the species was only found at a few rocky sites on the mountains and in valleys in moist locations near watercourses in the national park. It was found at three places along two transects where a total of 11 plants were counted (Mills 2012b).
The 2021 population estimate was 26 individuals.
The distribution is shown in Map 36.
Ecology
Monoecious. The plant propagates through division of the rather succulent stem and spreads as the stems produce roots on contact with the ground (Mills 2012b).
Habitat
This species occurs in damp shade within moist palm valley forest. It is found on cliffs behind shaded streams within the national park and is restricted to very steep rocky bands and cliffs in shaded valley bottoms where there is always adequate moisture (Sykes & Atkinson 1988). This species is affected by the erosion regime of the island—active down-cutting by streams might open new sites while heavy downpours might wipe out existing colonies (Sykes & Atkinson 1988).
Threats
The main threats to the species are weed invasion and competition—especially by weeds such William Taylor (Ageratina riparia) (TSSC 2003e)— and drought/dry conditions due to climate change.
Impact on other species
None known.
Map 36 Distribution of Elatostema montanum
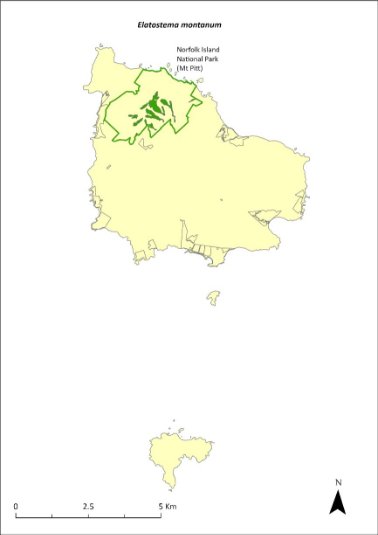
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021).
Risk assessment
Risk assessment undertaken for Critically Endangered herbs/grasses as a grouping. The risk assessment is shown in Table 89.
Table 89 Risk assessment for Critically Endangered herbs/grasses as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Rare (0–10%) | Negligible | Negligible |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Unlikely (11–25%) | Minor | Low |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Almost certain (91–100%) | Major | Extreme |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Moderate | High |
Management actions
Undertake targeted weed control and maintenance around existing plants, ensuring shaded areas are not opened too quickly. Undertake revegetation/habitat restoration. Undertake propagation from cuttings or seed and plantings within suitable areas.
Recovery target
The recovery target is shown in Table 90.
Table 90 Recovery target for Elatostema montanum
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Critically Endangered | 26 | 100% within the national park | 100 |
Relevant literature
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Gilmour PM & Helman CE (1989b) The Vegetation of Norfolk Island National Park. Report to the Australian National Parks and Wildlife Service, Norfolk Island.
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003e) Commonwealth Listing Advice—Critically Endangered Elatostema montanum (Mountain Procris, a herb).
Euphorbia norfolkiana—Norfolk Island euphorbia
Family EUPHORBIACEAE
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Critically Endangered.
Description
A dense low shrub usually growing to 1m but sometimes to 3m tall with olive green flowers.
Distribution and abundance
There were 38 mature individuals of Euphorbia norfolkiana known in 2003 (TSSC 2003a). A 2007 survey found a total of 104 plants on Norfolk Island, of which 42 plants were higher than 1m tall, including 12 plants in Bumbora Reserve and 5 plants in Ball Bay Reserve; most plants were on private land near Bumbora Reserve (Mills 2007d). In 2012 the species was decreasing in abundance, with only one small plant found in Bumbora Reserve, and 13 individuals counted on a transect in the valley east of Cook’s Monument in Norfolk Island National Park (Mills 2012b).
By 2021 the population had increased to 388, including 25 in Bumbora Reserve (planted in 2017) and 29 at Ball Bay Reserve (wild population) (Mills 2017a, b). The species has also been planted on Phillip Island (Mills 2009b).
Ecology
This species can establish on bare, loose soil but may need partial shade for the most effective establishment. Most plants establish below an open cover of pine.
Habitat
Occurs in in coastal pine and white oak forest (Invasive Species Council & TierraMar 2021), in open areas in light shade amidst coastal cliff vegetation, usually below pines.
Threats
Threats to the species include small population size and restricted distribution and subsequent increased risk of extinction through natural events such as cyclones, slips and drought; weed invasion and competition from weeds such as African olive (Olea europaea cuspidata) and kikuyu (Cenchrus clandestinus); and cattle grazing. Phytophthora cinnamomi is potentially a major risk.
Impact on other species
None known.
Map 37 Distribution of Euphorbia norfolkiana
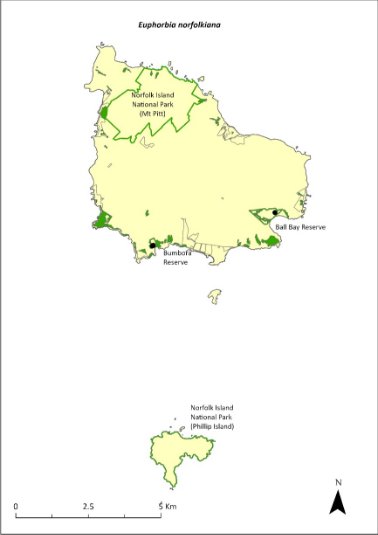
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021). Points show recorded locations (Mills 2017a, b).
Risk assessment
Risk assessment undertaken for Critically Endangered trees/shrubs as a grouping. The risk assessment is shown in Table 91.
Table 91 Risk assessment for Critically Endangered trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Almost certain (91–100%) | Major | Extreme |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Likely (51–90%) | Moderate | Medium |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Possible (26–50%) | Moderate | Medium |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Major | High |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Extreme | Extreme |
Management actions
Undertake targeted weed control and maintenance around existing plants. Undertake revegetation/habitat restoration. Exclude or manage cattle grazing. Undertake propagation and planting in shaded areas under pines in coastal habitat across all land tenures. Plants should be grown to a reasonable size before being planted out to increase survival rates. Investigate suitable habitat below pines within One Hundred Acres Reserve.
Monitor existing populations and conduct searches in other areas (such as adjacent to Bumbora reserve and along the coast to Beefsteak).
Recovery target
The recovery target is shown in Table 92.
Table 92 Recovery target for Euphorbia norfolkiana
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Critically Endangered | 388 | >85% within the national park >10% within public reserves | 1000 |
Relevant literature
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Mills K (2007d) The Flora of Norfolk Island. 5. Field Survey of the Norfolk Island Endemic Shrub Euphorbia norfolkiana (Euphorbiaceae). Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2009b) The Vegetation of Phillip Island, Norfolk Island Group. Envirofund 2007/2008. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2017a) Survey of public reserves on Norfolk Island for threatened plant species: 3. Point Ross Reserve and Bumbora Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017b) Survey of public reserves on Norfolk Island for threatened plant species: 8. Ball Bay Reserve. Prepared for Norfolk Island Regional Council.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003a) Commonwealth Listing Advice for Norfolk Island Flora – 11 Critically Endangered Species.
Euphorbia obliqua—a herb
Family EUPHORBIACEAE
Conservation significance
Australian distribution restricted to Norfolk Island. Also occurs in New Caledonia and Vanuatu.
EPBC Act Listing Status: Vulnerable.
Description
A prostrate perennial herb with stems to 20cm long.
Distribution and abundance
The natural population of Euphorbia obliqua is mostly found outside of the Norfolk Island National Park; there were 530 mature individuals known in 2003 (TSSC 2003b).
The species has been reported from Emily Bay, Kingston, the rocks near the Old Salt House, and The Chord at Duncombe Bay (Orchard 1994). It is common on Nepean Island, where Mills (2009a) found 154 plants, mainly along the southern shoreline. Mills (2017c) counted 814 in Point Hunter Reserve, the major population of the species on Norfolk Island, and 49 in Kingston Recreation Reserve.
The population estimate in 2021 was 814 individuals.
The distribution is shown in Map 38.
Ecology
Little known.
Habitat
Occurs within sandy beach herbland (Invasive Species Council & TierraMar 2021). Found in calcarenite cracks and fissures in coralline and sometimes basaltic rocks by the sea, with a woody rootstock penetrating the fissures.
Threats
E. obliqua is threatened by small population size and subsequent increased risk of extinction through natural events such as cyclones and drought, specific habitat requirements, and weed invasion and competition.
Impact on other species
None known.
Map 38 Distribution of Euphorbia obliqua
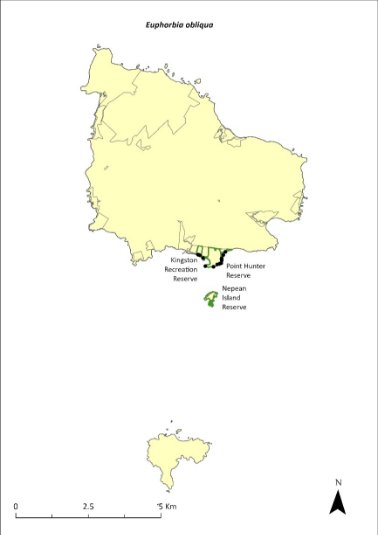
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Mills 2009a). Points show recorded locations (Mills2017c).
Risk assessment
Risk assessment undertaken for Vulnerable herbs/grasses as a grouping. The risk assessment is shown in Table 93.
Table 93 Risk assessment for Vulnerable herbs/grasses as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Rare (0–10%) | Negligible | Negligible |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Unlikely (11–25%) | Minor | Low |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Possible (26–50%) | Moderate | Medium |
Management actions
Undertake targeted weed control and maintenance. Undertake revegetation/habitat restoration. Exclude or manage cattle grazing. Monitor existing populations.
Recovery target
The recovery target is shown in Table 94.
Table 94 Recovery target for Euphorbia obliqua
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Vulnerable | 814 | <95% within the public reserves | 1500 |
Relevant literature
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Mills K (2009a) The Flora of Norfolk Island. 9. The Vegetation of Nepean Island (including Errata and Addenda for Papers 1 to 8). Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2017c) Survey of public reserves on Norfolk Island for threatened plant species: 1. The Kingston Reserves. Prepared for Norfolk Island Regional Council.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
TSSC (Threatened Species Scientific Committee) (2003b) Commonwealth Listing Advice for Norfolk Island Flora - 15 Vulnerable Species.
Hibiscus insularis—Phillip Island hibiscus
Family MALVACEAE
Conservation significance
Endemic to Phillip Island.
EPBC Act Listing Status: Critically Endangered.
Description
Large shrub growing to 2.5m high. The flowers are solitary and pale yellow with a greenish tinge, have a dark magenta centre, and turn purple as they age.
Distribution and abundance
There were 13 plants in 1939 and only eight surviving in 1963 (Orchard 1994). In 1988, Hibiscus insularis was restricted to one site only, with a major patch and a minor patch on the northern slopes of Phillip Island (Sykes & Atkinson 1988). In 2003 there were fewer than 50 mature plants surviving in the wild (TSSC 2003a). In 2009 there were over 100 plants on Phillip Island, with a large clump in the upper part of Long Valley (Mills 2009b).
Plants are now being cultivated at the Norfolk Island National Park Nursery, and the species has been planted in revegetation programs in public reserves and is grown in many private gardens. The population estimate in 2021 was 350 individuals.
The distribution is shown in Map 39.
Ecology
This species takes 18 years to mature from seed, but plants mature more quickly when propagated from cuttings.
The predatory moths Pectinophora scutigera and Anisoplaca cosmia may reduce reproductive success by destroying seeds (Groeneveld 1989).
H. insularis is pollinated by nectar feeding birds (Groeneveld 1989).
Habitat
Grows on the northern slopes of Phillip Island.
Threats
H. insularis is threatened by small population size and restricted distribution, and subsequent increased risk of extinction through natural events such as cyclones, slips and drought. Existing population has been totally derived from two individuals. Weed invasion and competition (particularly from African olive [Olea europaea cuspidata]) is a significant threat, which may be exacerbated by extended dry periods. Phytophthora cinnamomi is potentially a major risk.
Map 39 Distribution of Hibiscus insularis

Green outlines indicate reserves within which the species occurs. Points show recorded locations (Mills 2009b).
Impact on other species
None known.
Risk assessment
Risk assessment undertaken for Critically Endangered trees/shrubs as a grouping. The risk assessment is shown in Table 95.
Table 95 Risk assessment for Critically Endangered trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Likely (51–90%) | Moderate | Medium |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Likely (51–90%) | Moderate | Medium |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Possible (26–50%) | Minor | Low |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Minor | Low |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Moderate | High |
Management actions
Undertake propagation and planting within suitable areas on Phillip Island. Establish native tree shelter belts (such as white oaks) to replace the weed species that currently shelter the main population on Phillip Island. Undertake targeted weed control and ongoing maintenance, while maintaining a strict herbicide ban in the vicinity of the main population.
Recovery target
The recovery target is shown in Table 96.
Table 96 Recovery target for Hibiscus insularis
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Critically Endangered | 350 | >95% within the national park | 1000 |
Relevant literature
Groeneveld KM (1989) Conservation biology of the endangered species Hibiscus insularis. Unpublished Report to the Australian National Parks and Wildlife Service.
Mills K (2009b) The Vegetation of Phillip Island, Norfolk Island Group. Envirofund 2007/2008. Kevin Mills & Associates, Jamberoo, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003a) Commonwealth Listing Advice for Norfolk Island Flora – 11 Critically Endangered Species.
Hypolepis dicksonioides—downy ground fern, brake fern
Family DENNSTAEDTIACEAE
Conservation significance
Australian distribution is restricted to Norfolk and Phillip Islands but also occurs in the Kermadec Islands, New Zealand, Samoa, Society Islands and Marquesas.
EPBC Act Listing Status: Vulnerable.
Description
A terrestrial fern of disturbed sites with fronds growing to 100 cm or longer.
An important colonising fern that does not persist for long periods in the same location (Braggins 1996).
Distribution and abundance
This species occurs on Norfolk and Phillip Islands and has been recorded from Mt Bates (Orchard 1994). In a 1995 survey, it was reported from only one site outside of Phillip Island and was much less common than in 1971 (Brownsey & Chinnock 1987, Braggins 1996).
On Phillip Island, this fern is uncommon but widespread. It is most common in First West End Valley and the valleys across the eastern part of the island (Mills 2009b). Six clumps of the plant were also recorded from Bumbora Reserve (Mills 2017a). The number of mature individuals in 2003 was fewer than 500 (TSSC 2003b).
The distribution is shown in Map 40.
Ecology
Little known.
Habitat
Grows in disturbed sites and open rocky places.
Threats
The main threats to the species are weed invasion and competition, and small population size and subsequent increased risk of extinction through natural events such as cyclones, slips and drought. Drought/dry conditions due to climate change are also a threat.
Impact on other species
None known.
Map 40 Distribution of Hypolepis dicksonioides
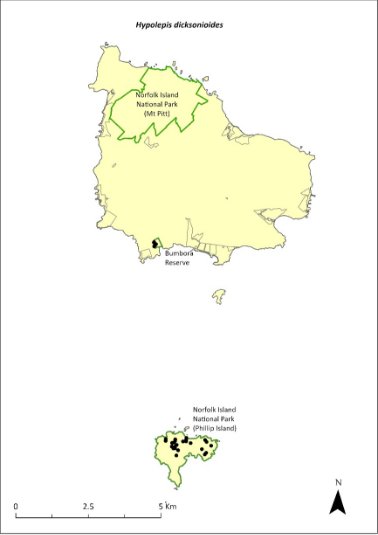
Green outlines indicate reserves within which the species occurs. Points show recorded locations (Mills 2009b and 2017a).
Risk assessment
Risk assessment undertaken for Vulnerable ferns as a grouping. The risk assessment is shown in Table 97.
Table 97 Risk assessment for Vulnerable ferns as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Rare (0–10%) | Negligible | Negligible |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Possible (26–50%) | Moderate | Medium |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Major | Extreme |
Management actions
Undertake targeted weed control and maintenance. Undertake revegetation/habitat restoration and propagation and planting within suitable areas.
Recovery target
The recovery target is shown in Table 98.
Table 98 Recovery target for Hypolepis dicksonioides
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Vulnerable | 506 | 99% within the national park 1% within public reserves | 750 |
Relevant literature
Braggins JE (1996) Report on the conservation status of the ferns of Norfolk Island. Unpublished report to the Australian Nature Conservation Agency.
Brownsey PJ & Chinnock RJ (1987) A taxonomic revision of the Australian species of Hypolepis. Journal of the Adelaide Botanical Garden 10, 1–30.
Mills K (2009b) The Vegetation of Phillip Island, Norfolk Island Group. Envirofund 2007/2008. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2017a) Survey of public reserves on Norfolk Island for threatened plant species: 3. Point Ross Reserve and Bumbora Reserve. Prepared for Norfolk Island Regional Council.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
TSSC (Threatened Species Scientific Committee) (2003b) Commonwealth Listing Advice for Norfolk Island Flora - 15 Vulnerable Species.
Ileostylus micranthus—mistletoe
Family LORANTHACEAE
Conservation significance
Ileostylus micranthus is best known from New Zealand and was first collected on Norfolk Island in the 1930s. It is presumed to be a recent arrival on Norfolk Island as previous collectors had failed to find it (Orchard 1994).
EPBC Act Listing Status: Vulnerable.
Description
A bushy epiphytic mistletoe with green flowers and yellow fruit.
Distribution and abundance
I. micranthus has been reported from the upper slopes of Mt Pitt and the track leading to Red Road on Mt Bates (Orchard 1994) above the 200-metre contour. On Norfolk Island the number of mature individuals was fewer than 500 in 2003 (TSSC 2003b).
The distribution is shown in Map 41.
Ecology
An epiphytic parasite with a wide range of host plants.
Habitat
Occurs scattered on several host species within the national park, favouring Coprosma pilosa as its host.
Threats
The species is threatened by weed invasion, catastrophic events (such as severe storms), and limited distribution of its host C. pilosa.
Impact on other species
Parasitic on host species and heavy infestations on C. pilosa can kill the host plant.
Map 41 Distribution of Ileostylus micranthus
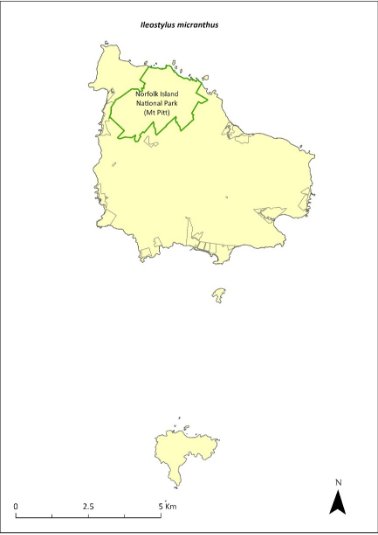
Green outlines indicate reserves within which the species occurs.
Risk assessment
The risk assessment is shown in Table 99.
Table 99 Risk assessment for Ileostylus micranthus
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Unlikely (11–25%) | Minor | Low |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Unlikely (11–25%) | Moderate | Low |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Unlikely (11–25%) | Minor | Low |
15. Problems caused by small populations, including lack of genetic diversity | Possible (26–50%) | Major | High |
Management actions
Undertake revegetation/habitat restoration. Undertake targeted weed control and maintenance.
Recovery target
The recovery target is shown in Table 100.
Table 100 Recovery target for Ileostylus micranthus
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Vulnerable | 500 | 100% within the national park | 750 |
Relevant literature
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
TSSC (Threatened Species Scientific Committee) (2003b) Commonwealth Listing Advice for Norfolk Island Flora - 15 Vulnerable Species.
Lastreopsis calantha—shield-fern
Family DRYOPTERIDACEAE
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Endangered.
The Australian Plant Census accepts Parapolystichum calanthum as a synonym.
Description
A terrestrial fern with a long creeping rhizome and fronds growing to 30cm, occasionally reaching 50cm long.
Distribution and abundance
Lastreopsis calantha is known to occur mainly in the Norfolk Island National Park, in damp and shady valleys (Sykes & Atkinson 1988).
Braggins (1996) found it at several widely scattered sites, and the species had returned in areas where cattle had been excluded. There were fewer than 200 mature individuals in 2003 (TSSC 2003c). Mills (2012b) found 148 plants along 10 transects, mostly within valleys. The valley to the south-west of Bird Rock in the national park contained the largest population of this fern, with 72 plants.
The distribution is shown in Map 42.
Ecology
Grows in moist shaded areas.
Habitat
Grows in moist upland hardwood forest, mainly in shaded valleys.
Threats
The main threats to the species are cattle grazing, weed invasion and changes to hydrology in the national park.
Impact on other species
None known.
Map 42 Distribution of Lastreopsis calantha
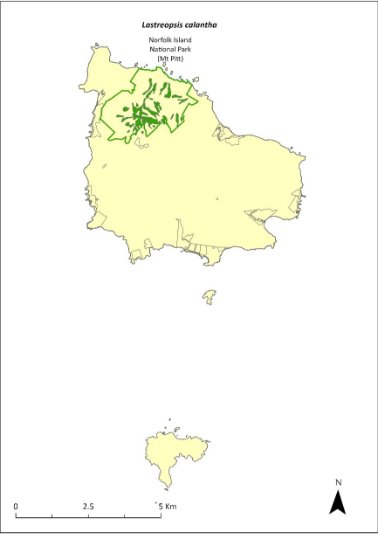
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021).
Risk assessment
Risk assessment undertaken for Endangered ferns as a grouping. The risk assessment is shown in Table 101.
Table 101 Risk assessment for Endangered ferns as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Rare (0–10%) | Negligible | Negligible |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Moderate | Medium |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Unlikely (11–25%) | Moderate | Low |
Management actions
Implement revegetation/habitat restoration. Undertake ongoing targeted weed control and maintenance. Exclude or manage cattle grazing. Monitor to determine population dynamics.
Recovery target
The recovery target is shown in Table 102.
Table 102 Recovery target for Lastreopsis calantha
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Endangered | 148 | 100% within the national park | 250 |
Relevant literature
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
Braggins JE (1996) Report on the conservation status of the ferns of Norfolk Island. Unpublished report to the Australian Nature Conservation Agency.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003c) Commonwealth Listing Advice for Norfolk Island Flora – 16 Endangered Species.
Marattia salicina (Ptisana salicina)—king fern, para, potato fern
Family MARATTIACEAE
Conservation significance
Australian distribution restricted to Norfolk Island; it is also found in New Zealand
EPBC Act Listing Status: Endangered
This species is listed under the EPBC Act as Marattia salicina. Now known as Ptisana salicina, Marattia salicina is also listed as a synonym.
Description
Large robust fern with fronds growing to 6m long.
Distribution and abundance
It was reported from several sites within the Norfolk Island National Park with some regeneration in 1996 (Braggins 1996). In 2003 there were fewer than 100 mature individuals, which were all within the Mt Pitt section of the national park (TSSC 2003c). Mills (2012b) recorded 44 plants in six valleys within the national park. The largest number of plants (33) were observed in King Fern Valley, to the east of Mount Pitt Road.
The population estimate in 2021 was 160 individuals.
In New Zealand it was listed as “chronically threatened—Serious Decline” (de Lange et al. 2004).
The distribution is shown in Map 43.
Ecology
Little known.
Habitat
This species grows in valleys on south-east mountain slopes (Orchard 1994).
Threats
Illegal collection from the national park has occurred in the past (Braggins 1996). Climate change/dry conditions are also a threat.
Impact on other species
None known.
Map 43 Distribution of Marattia salicina
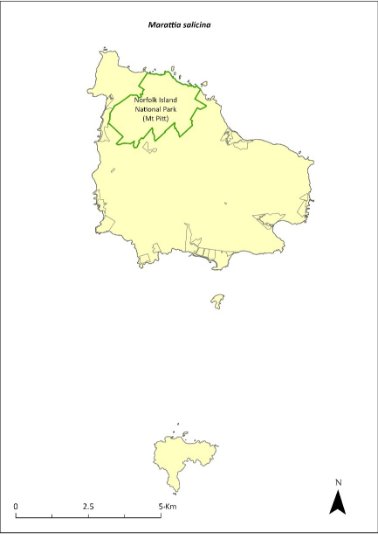
Green outlines indicate reserves within which the species occurs.
Risk assessment
Risk assessment undertaken for Endangered ferns as a grouping. The risk assessment is shown in Table 103.
Table 103 Risk assessment for Endangered ferns as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Rare (0–10%) | Negligible | Negligible |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Major | High |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Major | Extreme |
Management actions
Undertake propagation and revegetation in suitable habitat (including sales to private landholders to reduce pressure on naturally occurring populations). Protect and restore habitat. Investigate sites on public reserves for introduction.
Recovery target
The recovery target is shown in Table 104.
Table 104 Recovery target for Marattia salicina
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Endangered | 160 | 100% within the national park | 250 |
Relevant literature
Braggins JE (1996) Report on the conservation status of the ferns of Norfolk Island. Unpublished report to the Australian Nature Conservation Agency.
de Lange PJ, Johnson PN, Norton DA, Hitchmough R, Heenan PB, Courteney SP, Molloy B.P.J, Ogle C.C & Rance BD (2004) Threatened and uncommon plants of New Zealand. New Zealand Journal of Botany 42, 45–76.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003c) Commonwealth Listing Advice for Norfolk Island Flora – 16 Endangered Species.
Melicope littoralis—shade tree
Family RUTACEAE
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Vulnerable.
Description
Tree to 5 m tall, with trifoliolate leaves (having three leaflets), small creamy-white flowers and shiny black seeds.
Distribution and abundance
There were 273 mature individuals in 2003 with most plants occurring in the Mt Pitt section of the national park (Orchard 1994, TSSC 2003b). In 2010 it was considered widespread within the park and the 2021 population estimate was 305.
The distribution is shown in Map 44.
Ecology
Little known.
Habitat
Occurs in moist upland hardwood forest (Invasive Species Council & TierraMar 2021).
Threats
The black rat may contribute to the failed reproduction of this species (Bell 1990). Phytophthora cinnamomi is potentially a major risk.
Impact on other species
None known.
Map 44 Distribution of Melicope littoralis
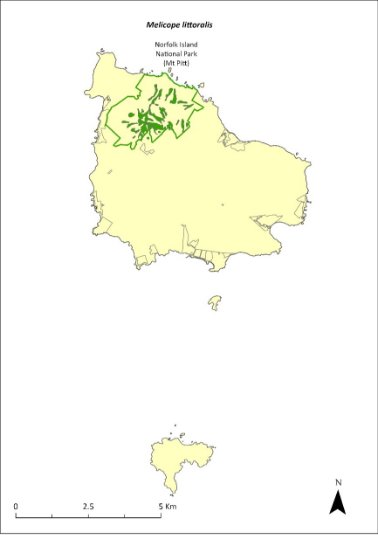
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021).
Risk assessment
Risk assessment undertaken for Vulnerable trees/shrubs as a grouping. The risk assessment is shown in Table 105.
Table 105 Risk assessment for Vulnerable trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Likely (51–90%) | Moderate | Medium |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Major | High |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Likely (51–90%) | Major | High |
Management actions
Undertake rodent control. Carry out targeted weed control and maintenance. Undertake propagation and planting within suitable areas. Undertake revegetation/habitat restoration.
Recovery target
The recovery target is shown in Table 106.
Table 106 Recovery target for Melicope littoralis
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Vulnerable | 305 | 100% within the national park | 1000 |
Relevant literature
Bell BD (1990) The status and management of the White-breasted White-eye and other birds of Norfolk Island. Unpublished report to the Australian Nature Conservation Agency.
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
TSSC (Threatened Species Scientific Committee) (2003b) Commonwealth Listing Advice for Norfolk Island Flora - 15 Vulnerable Species.
Melicytus latifolius—Norfolk Island mahoe
Family VIOLACEAE
Conservation significance
Endemic to Norfolk Island
EPBC Act Listing Status: Critically Endangered
Description
A small tree usually growing to 4m tall but sometimes reaching 9m.
Distribution and abundance
Melicytus latifolius is found in the valleys and on the slopes of the Mt Pitt section of Norfolk Island National Park, together with a few areas outside the park in the Mission Road area (Sykes & Atkinson 1988). 40 plants were identified in 1988, with a high proportion of juveniles and few seedlings or mature trees (Sykes & Atkinson 1988). Historical records suggest this plant has been rare for some time (Gilmour & Helman 1989b).
The last good flowering season was in the late 1990s/early 2000s when a large number of plants were produced in the natural forest and in the nursery plantings (Director of National Parks 2010). There were 17 known mature individuals in 2003 (TSSC 2003a). Surveys in 2012 (Mills 2012b) found a total of 16 plants along five transects, all saplings. More recently, searches have found over 100 new adults in the wild, which have been tagged for monitoring. The population estimate in 2021 was 148.
The distribution is shown in Map 45.
Ecology
Little known.
Habitat
This species requires moist shaded valley sites and broad ridges, and, while it can tolerate dense shade, it sometimes establishes at the edge of canopy gaps (Sykes & Atkinson 1988). It occurs in moist upland hardwood forest and plateau hardwood forest (Invasive Species Council & TierraMar 2021).
Threats
The main threats to the species are weed invasion and competition, particularly by red guava (Psidium cattleyanum) and wild tobacco (Solanum mauritianum). Unpredictable and irregular seed production is also a threat. Phytophthora cinnamomi is potentially a major risk.
Impact on other species
None known.
Map 45 Distribution of Melicytus latifolius

Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021).
Risk assessment
Risk assessment undertaken for Critically Endangered trees/shrubs as a grouping. The risk assessment is shown in Table 107.
Table 107 Risk assessment for Critically Endangered trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Possible (26–50%) | Major | High |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Likely (51–90%) | Moderate | Medium |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Almost certain (91–100%) | Major | Extreme |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Major | Extreme |
Management actions
Monitor seed production and collect and propagate seed when available. Conduct regular monitoring to determine causes of mortality. Carry out targeted weed control and maintenance and undertake replanting on moist slopes within the national park (Sykes & Atkinson 1988). Continue protection of the Mission Road rainforest remnants. Exclude or manage cattle grazing.
Recovery target
The recovery target is shown in Table 108.
Table 108 Recovery target for Melicytus latifolius
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Critically Endangered | 148 | >95% within the national park | 500 |
Relevant literature
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
Director of National Parks (2010) Norfolk Island Region Threatened Species Recovery Plan. Department of the Environment, Water, Heritage and the Arts, Canberra.
Gilmour PM & Helman CE (1989b) The Vegetation of Norfolk Island National Park. Report to the Australian National Parks and Wildlife Service, Norfolk Island.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003a) Commonwealth Listing Advice for Norfolk Island Flora – 11 Critically Endangered Species.
Melicytus ramiflorus subsp. oblongifolius—whiteywood
Family VIOLACEAE
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Vulnerable.
Description
A slender shrub or tree to 5m tall.
Distribution and abundance
This subspecies has been recorded on the saddle between Mt Pitt and Mt Bates in the Norfolk Island National Park, and north-east of the Kingston Cemetery (Orchard 1994). There were 436 mature individuals recorded in 2003 (TSSC 2003b).
It is relatively widespread in the national park, occurring in viny hardwood forest on the south‑western flanks of the mountains (Invasive Species Council & TierraMar 2021). There are also a few individuals within Ball Bay and Hundred Acres Reserves (Mills 2017b).
The population estimate in 2021 was 570.
The distribution is shown in Map 46.
Ecology
Little known.
Habitat
Forest.
Threats
Major threats to the species are cattle grazing, weed invasion and competition, and drought/dry conditions due to climate change. Phytophthora cinnamomi is potentially a major risk.
Impact on other species
None known.
Map 46 Distribution of Melicytus ramiflorus subsp. oblongifolius
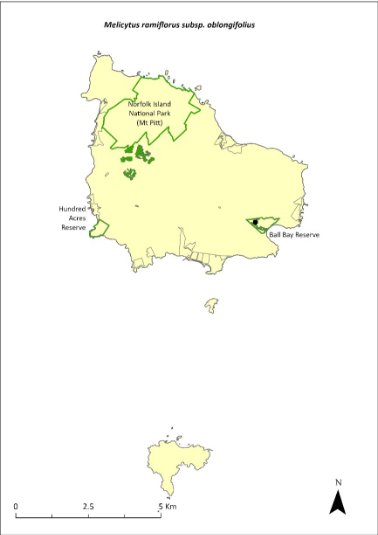
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021). Points show recorded locations (Mills 2017b).
Risk assessment
Risk assessment undertaken for Vulnerable trees/shrubs as a grouping. The risk assessment is shown in Table 109.
Table 109 Risk assessment for Vulnerable trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Likely (51–90%) | Moderate | Medium |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Almost certain (91–100%) | Moderate | High |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Possible (26–50%) | Moderate | Medium |
Management actions
Undertake propagation and planting within suitable areas, including public reserves. Implement habitat protection and rehabilitation. Carry out ongoing targeted weed control and maintenance. Exclude or manage cattle grazing.
Recovery target
The recovery target is shown in Table 110.
Table 110 Recovery target for Melicytus ramiflorus subsp. oblongifolius
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Vulnerable | 570 | 99% within the national park 1% within public reserves | 1000 |
Relevant literature
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Mills K (2017b) Survey of public reserves on Norfolk Island for threatened plant species: 8. Ball Bay Reserve. Prepared for Norfolk Island Regional Council.
TSSC (Threatened Species Scientific Committee) (2003b) Commonwealth Listing Advice for Norfolk Island Flora - 15 Vulnerable Species.
Meryta angustifolia—Narrow-leaved Meryta
Family ARALIACEAE
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Vulnerable.
Description
Tree growing to 6m tall with few branches.
Distribution and abundance
There were 479 mature individuals of Meryta angustifolia recorded on Norfolk Island in 2003 (TSSC 2003b). Orchard (1994) reported that the species was widespread within the Norfolk Island National Park (particularly in areas where weed control had been carried out) and recorded it from the northern and southern slopes of Mt Bates, on the saddle between Mt Pitt and Mt Bates, and from outside the national park. Most of the population is within the park but there are some individuals in the Mission Road rainforest remnants.
The population estimate in 2021 was 494.
The distribution is shown in Map 47.
Ecology
Little known.
Habitat
Occurs in moist upland hardwood forest and pine-hardwood ridge forest (Invasive Species Council & TierraMar 2021).
Threats
Threats to the species include cattle grazing, weed invasion, and limited seed production in comparison with M. latifolia. Phytophthora cinnamomi is potentially a major risk.
Impact on other species
None known.
Map 47 Distribution Meryta angustifolia
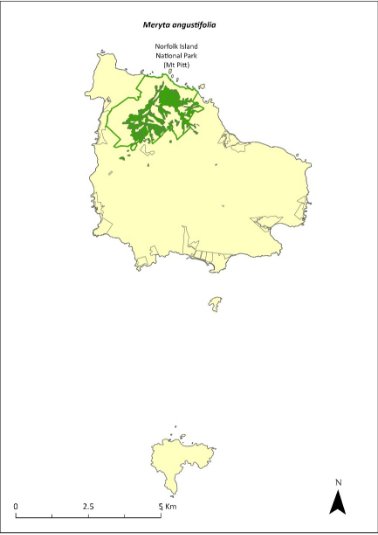
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021).
Risk assessment
Risk assessment undertaken for Vulnerable trees/shrubs as a grouping. The risk assessment is shown in Table 111.
Table 111 Risk assessment for Vulnerable trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Almost certain (91–100%) | Major | Extreme |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Moderate | Medium |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Possible (26–50%) | Moderate | Medium |
Management actions
Undertake propagation and planting within suitable areas, including the public reserves. Implement habitat protection and rehabilitation. Conduct targeted weed control and maintenance. Maintain fencing and current grazing exclusion in Mission Road rainforest remnants.
Recovery target
The recovery target is shown in Table 112.
Table 112 Recovery target for Meryta angustifolia
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Vulnerable | 494 | >95% within the national park | 1000 |
Relevant literature
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
TSSC (Threatened Species Scientific Committee) (2003b) Commonwealth Listing Advice for Norfolk Island Flora - 15 Vulnerable Species.
Meryta latifolia—broad-leaved meryta
Family ARALIACEAE
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Critically Endangered.
Description
Very distinctive tree with large, very broad, dark green leaves growing to 6m tall with few branches and producing a large yellow flower spike.
Distribution and abundance
Only 33 plants were found in 1988; 20 of these were in the Mission Road rainforest remnants (Sykes & Atkinson 1988). While the total number of mature individuals of Meryta latifolia in 2003 was 149, the effective reproductive population was determined by the limited number of mature female plants, which was estimated to be approximately 20 (TSSC 2003f). In 2012, 110 plants were counted within the national park, almost all of which were along tracks and on valley floors where they had been planted (Mills 2012b).
The species is now found on Phillip Island where it has been planted in various locations amongst Norfolk Island pine and white oak (Mills 2009b), and it also occurs in public reserves and in many private gardens on Norfolk Island. Propagation and planting have occurred through the Norfolk Island National Park threatened flora program.
The population estimate in 2021 was 395, including 216 in Anson Bay and Selwyn Reserve (Mills 2017d).
The distribution is shown in Map 48.
Ecology
Little known.
Habitat
This species grows on steep slopes, coastal cliffs, and within both shaded and unshaded sites. It is most commonly found at the edges of canopy gaps or along forest margins but can sometimes be found beneath Norfolk Island pines (Araucaria heterophylla; Sykes & Atkinson 1988). It occurs in viny hardwood forest and sheltered coastal forest (Invasive Species Council & TierraMar 2021).
Map 48 Distribution of Meryta latifolia
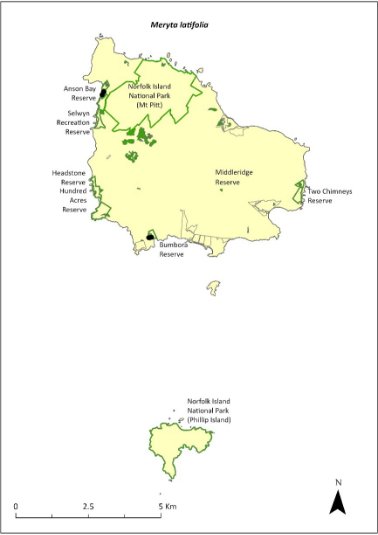
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021). Points show recorded locations (Mills 2017d).
Threats
Threats to the species include weed invasion, predation of seeds by rats, senescence of over-mature plants, sex ratio bias and cattle grazing. The species is adapted to moist forest conditions and is therefore susceptible to climate change (TSSC 2003f). Phytophthora cinnamomi is potentially a major risk.
Impact on other species
None known.
Risk assessment
Risk assessment undertaken for Critically Endangered trees/shrubs as a grouping. The risk assessment is shown in Table 113.
Table 113 Risk assessment for Critically Endangered trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Almost certain (91–100%) | Major | Extreme |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Likely (51–90%) | Moderate | Medium |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Moderate | Medium |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Major | Extreme |
Management action
Continue seed collection, propagation and replanting, including into lowland and coastal areas that are protected from cattle grazing (Sykes & Atkinson 1988). Implement habitat protection and rehabilitation. Undertake ongoing targeted weed control and maintenance. Maintain fencing and current grazing exclusion in Mission Road rainforest remnants and in public reserves. Facilitate additional plantings in private gardens.
Recovery target
The recovery target is shown in Table 114.
Table 114 Recovery target for Meryta latifolia
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Critically Endangered | 395 | >25% within the national park >70% within public reserves | 1000 |
Relevant literature
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Mills K (2009b) The Vegetation of Phillip Island, Norfolk Island Group. Envirofund 2007/2008. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2017d) Survey of public reserves on Norfolk Island for threatened plant species: 6. Anson Bay Reserve and Selwyn Reserve. Prepared for Norfolk Island Regional Council.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003f) Commonwealth Listing Advice—Critically Endangered Meryta latifolia (Shade Tree).
Muehlenbeckia australis—shrubby creeper, pohuehue
Family POLYGONACEAE
Conservation significance
Restricted to Norfolk Island and New Zealand.
EPBC Act Listing Status: Endangered.
Description
Perennial, much-branched, scrambling creeper that grows to 4 m high.
There may be genetic differences between the Norfolk and the Phillip Island population.
Distribution and abundance
This species occurs from sea level to the summit of Mt Pitt and has been recorded from Mt Pitt, Mt Bates and Steels Point (Orchard 1994). It occurs mostly within the Mt Pitt section of the Norfolk Island National Park, but also occurs on Phillip Island on the cliffs east of Razorback (Mills 2009b).
There were 100 mature individuals of Muehlenbeckia australis recorded in 2003 (TSSC 2003c). In 2012, Mills found 31 plants (most of which were small) along seven transects within the national park. The plants mainly occurred on ridges on the higher mountains. More than half the plants observed (17) were seen along the edge of the Mount Pitt Road (Mills 2012b).
The distribution is shown in Map 49.
Ecology
Little known.
Habitat
Grows in native forests from sea level to the upper slopes of the mountains. Usually seen in light gaps or on the edges of forest.
Threats
Threats include weed invasion and difficulty in propagating from seed.
Impact on other species
None known.
Map 49 Distribution of Muehlenbeckia australis
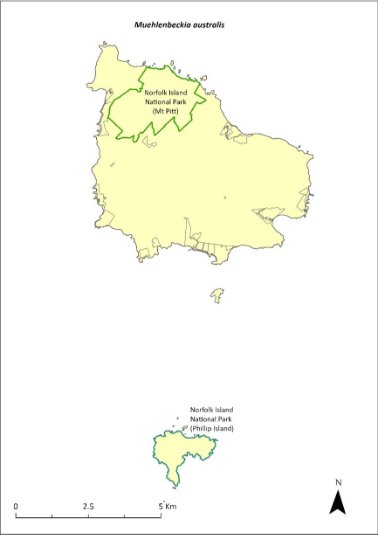
Green outlines indicate reserves within which the species occurs.
Risk assessment
Risk assessment undertaken for Endangered vines/climbers as a grouping. The risk assessment is shown in Table 115.
Table 115 Risk assessment for Endangered vines/climbers as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Possible (26–50%) | Minor | Low |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Moderate | Medium |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Possible (26–50%) | Moderate | Medium |
Management actions
Undertake propagation from cuttings from individuals on Norfolk Island and Phillip Island and replanting into appropriate areas. Conduct research into seed propagation techniques. Implement habitat protection and rehabilitation. Undertake targeted weed control and maintenance. Undertake research into taxonomic differences between the populations from Phillip Island and Norfolk Island and compare with those from New Zealand.
Recovery target
The recovery target is shown in Table 116.
Table 116 Recovery target for Muehlenbeckia australis
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Endangered | 100 | 100% within the national park | 250 |
Relevant literature
Mills K (2009b) The Vegetation of Phillip Island, Norfolk Island Group. Envirofund 2007/2008. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
TSSC (Threatened Species Scientific Committee) (2003c) Commonwealth Listing Advice for Norfolk Island Flora – 16 Endangered Species.
Myoporum obscurum—popwood
Family MYOPORACEAE
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Critically Endangered.
Description
Shrub or small spreading tree growing to 7 m tall with shiny green foliage.
Distribution and abundance
In 1988, the species was found on the northern slopes of the Norfolk Island National Park and on Mt Bates Road, but only four trees were sighted. It was not present on Phillip Island, although it was recorded there in the 1830s (Sykes & Atkinson 1988). A survey in 1989 failed to locate additional individuals again, confirming that this species was very rare in the national park (Gilmour & Helman 1989b). There were only five mature, seed-producing individuals of Myoporum obscurum remaining until replanting began in 1995.
Mills (2012b) found the species during surveys within the park at four sites where six plants were counted. No seedlings were found and only two wild trees were found at two of the sites surveyed. The species has been planted along the tracks in the national park and in gardens on Norfolk Island. The species was recorded within several reserves in 2017 (mostly plantings), including Selwyn, Cascade and Two Chimneys Reserves (Mills 2017d, f, g).
The population had increased to 417 individuals in 2021. Propagation and planting have occurred through the Norfolk Island National Park threatened flora program.
The distribution is shown in Map 50.
Ecology
Little known.
Habitat
This species prefers clearings, canopy gaps or open areas away from the coast (Sykes & Atkinson 1988).
Threats
Threats to the species include weed invasion and competition, and small population size and subsequent increased risk of extinction through natural events such as cyclones, slips and drought. Phytophthora cinnamomi is potentially a major risk.
Map 50 Distribution of Myoporum obscurum

Green outlines indicate reserves within which the species occurs. Points show recorded locations (Mills 2017a, d, e, f, g).
Impact on other species
None known.
Risk assessment
Risk assessment undertaken for Critically Endangered trees/shrubs as a grouping. The risk assessment is shown in Table 117.
Table 117 Risk assessment for Critically Endangered trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Unlikely (11–25%) | Minor | Low |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Likely (51–90%) | Moderate | Medium |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Major | High |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Major | Extreme |
Management actions
Continue propagation and replanting into appropriate areas on Norfolk Island and Phillip Island, as well as the public reserves. Implement habitat protection and rehabilitation. Undertake targeted weed control and maintenance. Monitor plantings to determine survival rates.
Recovery target
The recovery target is shown in Table 118.
Table 118 Recovery target for Myoporum obscurum
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Critically Endangered | 417 | >90% within the national park 4% within the Reserves | 1000 |
Relevant literature
Gilmour PM & Helman CE (1989b) The Vegetation of Norfolk Island National Park. Report to the Australian national parks and Wildlife Service, Norfolk Island.
Mills K (2009b) The Vegetation of Phillip Island, Norfolk Island Group. Envirofund 2007/2008. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Mills K (2017a) Survey of public reserves on Norfolk Island for threatened plant species: 3. Point Ross Reserve and Bumbora Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017d) Survey of public reserves on Norfolk Island for threatened plant species: 6. Anson Bay Reserve and Selwyn Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017e) Survey of public reserves on Norfolk Island for threatened plant species: 7. Hundred Acres Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017f) Survey of public reserves on Norfolk Island for threatened plant species: 4. Cascade Reserve including Quarantine Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017g) Survey of public reserves on Norfolk Island for threatened plant species: 5. Two Chimneys Reserve. Prepared for Norfolk Island Regional Council.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003a) Commonwealth Listing Advice for Norfolk Island Flora – 11 Critically Endangered Species.
Myrsine ralstoniae—beech
Family MYRSINACEAE
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Vulnerable.
Description
A small tree growing to 6 m tall with small berries.
Distribution and abundance
Most of the Myrsine ralstoniae population is within the Mt Pitt section of the Norfolk Island National Park. The species is widespread and very common in the national park. Elsewhere, it occurs in most public reserves and in forest remnants on private land (K Mills 2024. pers comm 11 January).
The total number of mature plants present in 2003 was 562 (TSSC 2003b). The population estimate in 2021 was 1789, including 409 in Anson Bay and Selwyn Reserves and 547 in Hundred Acres Reserve (Mills 2017d & e).
The distribution is shown in Map 51.
Ecology
Little known.
Habitat
The species often occurs as an understorey tree in forested areas (Orchard 1994). Occurs in moist upland hardwood forest, pine-hardwood ridge forest, viny hardwood forest, plateau hardwood forest, lowland valley hardwood forest, sheltered coastal forest and coastal pine and white oak forest (Invasive Species Council & TierraMar 2021).
Threats
Threats to the species include weed invasion and competition, cattle grazing and climate change. Phytophthora cinnamomi is potentially a major risk.
Impact on other species
None known.
Map 51 Distribution of Myrsine ralstoniae
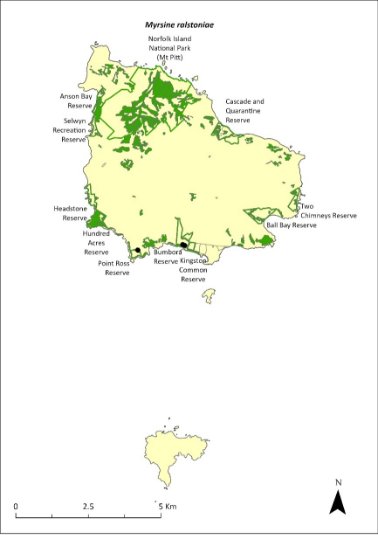
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021). Points show recorded locations (Mills 2017a and c).
Risk assessment
Risk assessment undertaken for Vulnerable trees/shrubs as a grouping. The risk assessment is shown in Table 119.
Table 119 Risk assessment for Vulnerable trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Almost certain (91–100%) | Moderate | High |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Minor | Medium |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Minor | Low |
15. Problems caused by small populations, including lack of genetic diversity | Unlikely (11–25%) | Minor | Low |
Management actions
Undertake seed collection, propagation and replanting. Implement habitat protection and rehabilitation. Undertake targeted weed control and maintenance. Exclude or manage cattle grazing.
Recovery target
The recovery target is shown in Table 120.
Table 120 Recovery target for Myrsine ralstoniae
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Vulnerable | 1789 | >30% within the national park >65% within public reserves | 3000 |
Relevant literature
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Mills K (2017d) Survey of public reserves on Norfolk Island for threatened plant species: 6. Anson Bay Reserve and Selwyn Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017e) Survey of public reserves on Norfolk Island for threatened plant species: 7. Hundred Acres Reserve. Prepared for Norfolk Island Regional Council.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
TSSC (Threatened Species Scientific Committee) (2003b) Commonwealth Listing Advice for Norfolk Island Flora - 15 Vulnerable Species.
Pennantia endlicheri—pennantia
Family ICACINACEAE
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Endangered
Description
A large shrub or more usually a tree growing to 10 m tall with small white flowers.
Distribution and abundance
It was estimated that a few hundred mature trees and about 3,000 saplings were present in the Mt Pitt section of the Norfolk Island National Park in the early 2000s (Director of National Parks 2010). The species is occasionally found outside of the park (Gardner & de Lange 2002; de Lange & Murray 2003). Some large trees are also known to occur in the Mission Road remnants (Director of National Parks 2010).
The total number of mature plants recorded within the national park in 2003 was 168 (TSSC 2003c). Mills (2012b) found that this species had greatly increased its population in the national park since 2003. A total of 680 plants were counted during the study on nearly all transects and ranged from seedlings to large old trees, indicating this species is increasing in abundance and is secure.
The population estimate in 2021 was 791 individuals. Propagation and planting have occurred through the Norfolk Island National Park threatened flora program.
The distribution is shown in Map 52.
Ecology
Flowers functionally unisexual.
Habitat
This species grows in sheltered moist palm valley forest, moist upland hardwood forest and pine‑hardwood ridge forest (Invasive Species Council & TierraMar 2021). Young plants have some shade tolerance such that they are often found in gullies dominated by Norfolk Island palms (Rhopalostylis baueri; Gardner & de Lange 2002).
Threats
Threats to the species include weed invasion and competition and rodents eating seeds. Phytophthora cinnamomi is potentially a major risk.
Impact on other species
None known.
Map 52 Distribution of Pennantia endlicheri
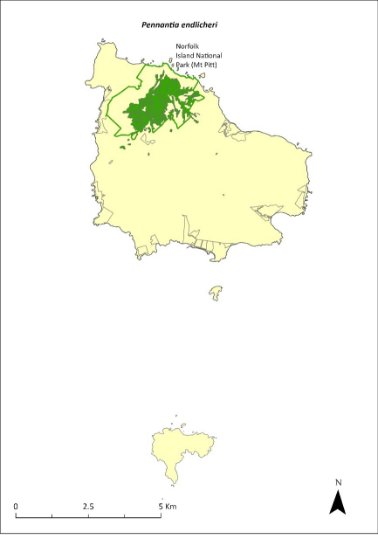
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021).
Risk assessment
Risk assessment undertaken for Endangered trees/shrubs as a grouping. The risk assessment is shown in Table 121.
Table 121 Risk assessment for Endangered trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Almost certain (91–100%) | Moderate | High |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Likely (51–90%) | Moderate | Medium |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Almost certain (91–100%) | Major | Extreme |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Moderate | High |
Management actions
Continue propagation and planting in suitable areas. Implement habitat protection and rehabilitation. Undertake ongoing targeted weed control and maintenance and ongoing rodent and chicken control.
Recovery target
The recovery target is shown in Table 122.
Table 122 Recovery target for Pennantia endlicheri
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Endangered | 791 | >95% within the national park | 1000 |
Relevant literature
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
de Lange PJ & Murray BG (2003) Chromosome numbers of Norfolk Island endemic plants. Australian Journal of Botany 51, 211–215.
Director of National Parks (2010) Norfolk Island Region Threatened Species Recovery Plan. Department of the Environment, Water, Heritage and the Arts, Canberra.
Gardner RO & de Lange PJ (2002) Revision of Pennantia (Icacinaceae), a small isolated genus. Journal of the Royal Society of New Zealand 32, 669–695.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
TSSC (Threatened Species Scientific Committee) (2003c) Commonwealth Listing Advice for Norfolk Island Flora – 16 Endangered Species.
Phreatia limenophylax—Norfolk Island phreatia
Family ORCHIDACEAE
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Critically Endangered.
Description
A small, tufted, epiphytic orchid growing to 3-6 cm high with a long inflorescence of many tiny greenish-white flowers.
Distribution and abundance
The species has been recorded from Anson Bay (Orchard 1994) but likely only remains within the Mt Pitt section of Norfolk Island National Park. The total number of mature Phreatia limenophylax plants in 2003 was five (TSSC 2003a). The species was not found during a 2007 study of epiphytes on Norfolk Island by Mills (2007e).
The distribution is shown in Map 53.
Ecology
Little known.
Habitat
Grows on the branches of trees in the Mt Pitt section of the national park, within moist palm valley forest.
Threats
Threats to the species include small population size and subsequent increased risk of extinction through natural events such as cyclones, slips and drought, and climate change.
Impact on other species
Grows on the branches of trees.
Map 53 Distribution of Phreatia limenophylax
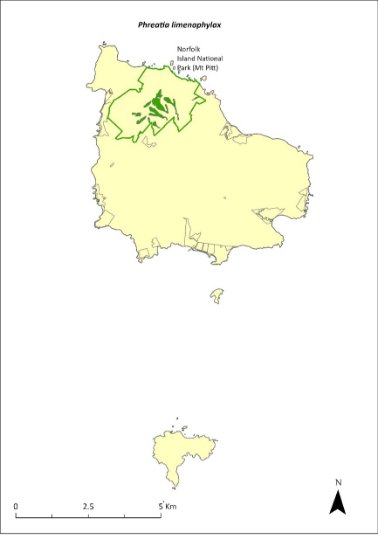
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021).
Risk assessment
Risk assessment undertaken for all threatened orchids as a grouping. The risk assessment is shown in Table 123.
Table 123 Risk assessment for all threatened orchids as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Minor | Medium |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Moderate | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Minor | Medium |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Rare (0–10%) | Negligible | Negligible |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Possible (26–50%) | Moderate | Medium |
12. Infection by pathogens already present | Unlikely (11–25%) | Moderate | Low |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Moderate | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Likely (51–90%) | Major | High |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Moderate | High |
Management actions
This orchid may require development of species-specific conservation actions, including ex situ conservation. Undertake surveys in suitable habitat areas to search for additional individuals/populations. Undertake ongoing targeted weed control and maintenance. Implement habitat protection and rehabilitation. Undertake research into the ecology of the species. Monitor/survey likely areas of the national park after storms, rescue any fallen specimens and attempt to cultivate them in the Norfolk Island National Park Nursery (Sykes & Atkinson 1988).
Recovery target
The recovery target is shown in Table 124.
Table 124 Recovery target for Phreatia limenophylax
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Critically Endangered | 5 | 100% within the national park | Established in a second location |
Relevant literature
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
Gilmour PM & Helman CE (1989b) The Vegetation of Norfolk Island National Park. Report to the Australian national parks and Wildlife Service, Norfolk Island.
Mills K (2007e) The Flora of Norfolk Island. 2. Epiphytes and Mistletoes. Kevin Mills & Associates, Jamberoo, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003a) Commonwealth Listing Advice for Norfolk Island Flora – 11 Critically Endangered Species.
Phreatia paleata—White lace orchid
Family ORCHIDACEAE
Conservation significance
Australian distribution restricted to Norfolk Island; also occurs in New Caledonia, New Guinea, Solomon Islands and Vanuatu (Orchard 1994).
EPBC Act Listing Status: Endangered
Description
A tufted epiphytic orchid growing to 30 cm high with 50 or more small white flowers on a drooping stem to 35 cm long.
Distribution and abundance
The population in 2003 consisted of fewer than 200 mature plants (TSSC 2003c).
The study of epiphytes on Norfolk Island by Mills (2007e) recorded 27 plants growing on trees and rocks, all in the national park, particularly at the higher altitudes.
The distribution is shown in Map 54.
Ecology
An epiphyte that grows on several tree species, apparently favouring Dysoxylum bijugum (Mills 2007e).
Habitat
Grows on tree branches in native forest.
Threats
Threats to the species include small population size and subsequent increased risk of extinction through natural events such as cyclones, slips and droughts, and climate change.
Impact on other species
Grows on the branches of other trees.
Map 54 Distribution of Phreatia paleata
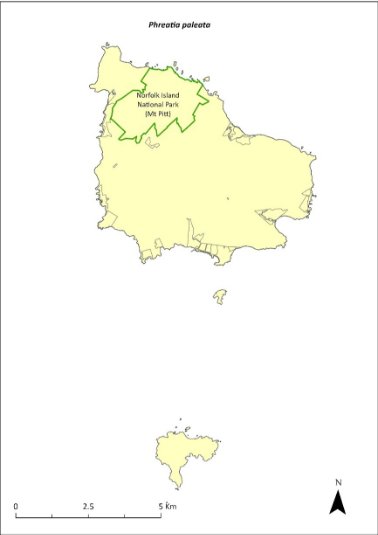
Green outlines indicate reserves within which the species occurs.
Risk assessment
Risk assessment undertaken for all threatened orchids as a grouping. The risk assessment is shown in Table 125.
Table 125 Risk assessment for all threatened orchids as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Minor | Medium |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Moderate | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Minor | Medium |
4. Degradation of native vegetation through current or future grazing | Unlikely (11–25%) | Moderate | Low |
6. Predation by rodents | Possible (26–50%) | Extreme | High |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Possible (26–50%) | Moderate | Medium |
12. Infection by pathogens already present | Unlikely (11–25%) | Moderate | Low |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Moderate | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Likely (51–90%) | Major | High |
15. Problems caused by small populations, including lack of genetic diversity | Likely (51–90%) | Moderate | Medium |
Management actions
Phreatia paleata may require development of species-specific conservation actions, including ex situ conservation. Undertake surveys in suitable habitat areas to search for additional individuals/populations. Undertake ongoing targeted weed control and maintenance, habitat protection and rehabilitation. Undertake research into the ecology of the species. Monitor likely areas of the national park after storms, rescue any fallen specimens and attempt to cultivate them in the Norfolk Island National Park Nursery (Sykes & Atkinson 1988).
Recovery target
The recovery target is shown in Table 126.
Table 126 Recovery target for Phreatia paleata
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Endangered | 27 | 100% within the national park | No decline |
Relevant literature
Mills K (2007e) The Flora of Norfolk Island. 2. Epiphytes and Mistletoes. Kevin Mills & Associates, Jamberoo, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003c) Commonwealth Listing Advice for Norfolk Island Flora – 16 Endangered Species.
Pittosporum bracteolatum—oleander
Family PITTOSPORACEAE
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Vulnerable.
Description
Tree growing to 7 m tall with small cream flowers and spherical fruit containing numerous seeds in a sticky orange pulp.
Distribution and abundance
Pittosporum bracteolatum is widespread with most of the wild population found in the Mt Pitt section of the Norfolk Island National Park. Elsewhere, it has been found at Mission Road, Steels Point and north-east of Bloody Bridge (Orchard 1994, Cayzer et al. 2000).
The total number of mature plants recorded in 2003 was 921 (TSSC 2003b). This species seeds well and it has been widely planted in the national park and in public reserves. The population estimate in 2021 was 1127 individuals, including 208 in Hundred Acres Reserve (Mills 2017e).
The distribution is shown in Map 55.
Ecology
Little known.
Habitat
Occurs in moist upland hardwood forest, pine-hardwood ridge forest and viny hardwood forest (Invasive Species Council & TierraMar 2021). It is quite common on parkland and forest slopes, particularly the sheltered south-east slopes of Mt Pitt (Cayzer et al. 2000).
Threats
The primary threat to the species is weed invasion and competition including from the introduced P. undulatum. Phytophthora cinnamomi is potentially a major risk.
Impact on other species
None known.
Map 55 Distribution of Pittosporum bracteolatum
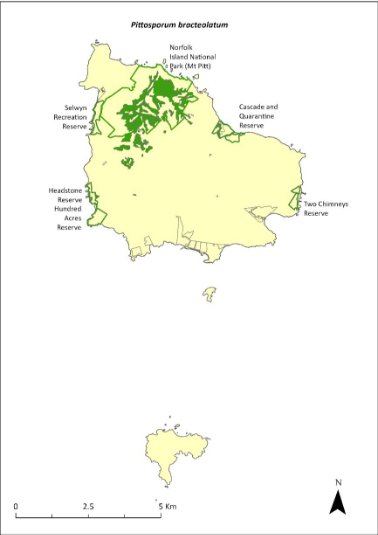
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021).
Risk assessment
Risk assessment undertaken for Vulnerable trees/shrubs as a grouping. The risk assessment is shown in Table 127.
Table 127 Risk assessment for Vulnerable trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Almost certain (91–100%) | Moderate | High |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Minor | Medium |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Unlikely (11–25%) | Minor | Low |
Management actions
Continue propagation and planting in suitable areas. Undertake targeted weed control and maintenance, with a focus on P. undulatum. Implement habitat protection and rehabilitation.
Recovery target
The recovery target is shown in Table 128.
Table 128 Recovery target for Pittosporum bracteolatum
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Vulnerable | 1349 | >80% within the national park >15% within the Reserves | 3000 |
Relevant literature
Cayzer LW, Crisp MD & Telford IRH (2000) A revision of Pittosporum (Pittosporaceae) in Australia. Systematic Journal of Botany 13, 845–902.
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Mills K (2017e) Survey of public reserves on Norfolk Island for threatened plant species: 7. Hundred Acres Reserve. Prepared for Norfolk Island Regional Council.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
TSSC (Threatened Species Scientific Committee) (2003b) Commonwealth Listing Advice for Norfolk Island Flora - 15 Vulnerable Species.
Planchonella costata—bastard ironwood
Family SAPOTACEAE
Conservation significance
Norfolk Island and New Zealand.
EPBC Act Listing Status: Endangered
Description
A small tree to 15m tall, which produces large (2.5–4 cm) multi-coloured berries and exudes a sticky white latex when wounded.
Distribution and abundance
Planchonella costata has been recorded from the slopes of Mt Pitt in the Norfolk Island National Park (Orchard 1994), in the Mission Road rainforest remnants and the botanic garden, and on private land at Simons Water.
There were 176 mature individuals recorded in the national park in 2003 (TSSC 2003c). Mills (2012b) found 34 plants on six transects within the national park, though the locations where this species is most common (lower altitudes and often on private property) were not surveyed. Mills (2017e) counted one planted individual in Hundred Acres Reserve.
The population estimate in 2021 was 251 individuals. Propagation and planting have occurred through the Norfolk Island National Park threatened flora program.
The distribution is shown in Map 56.
Ecology
Little known.
Habitat
A lowland species that is rare to absent at higher altitudes (K Mills 2024. pers comm 11 January). Grows in most types of forest except extreme/harsh coastal communities (Sykes & Atkinson 1988).
Threats
The primary threat to the species is weed invasion and competition. Phytophthora cinnamomi is potentially a major risk.
Impact on other species
None known.
Map 56 Distribution of Planchonella costata

Green outlines indicate reserves within which the species occurs.
Risk assessment
Risk assessment undertaken for Endangered trees/shrubs as a grouping. The risk assessment is shown in Table 129.
Table 129 Risk assessment for Endangered trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Possible (26–50%) | Minor | Low |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Likely (51–90%) | Moderate | Medium |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Major | High |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Likely (51–90%) | Major | High |
Management actions
Continue propagation and planting in suitable areas. Implement habitat protection and rehabilitation. Undertake ongoing targeted weed control and maintenance to promote natural regeneration.
Recovery target
The recovery target is shown in Table 130.
Table 130 Recovery target for Planchonella costata
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Endangered | 251 | >95% within the national park <1% within public reserves | 1000 |
Relevant literature
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2017e) Survey of public reserves on Norfolk Island for threatened plant species: 7. Hundred Acres Reserve. Prepared for Norfolk Island Regional Council.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003c) Commonwealth Listing Advice for Norfolk Island Flora – 16 Endangered Species.
Polyphlebium endlicherianum—middle filmy fern
Family HYMENOPHYLLACEAE
Conservation significance
Australian distribution is restricted to Norfolk Island and Queensland. Also known from New Zealand, Fiji and Vanuatu, east to Samoa and Tahiti.
EPBC Act Listing Status: Endangered.
For further information on the species outside of the Norfolk Island Group, see the species profile on SPRAT.
Description
Delicate epiphytic, lithophytic or terrestrial fern with small fronds growing to 10cm long.
Distribution and abundance
There were fewer than 200 mature individuals in the wild recorded on Norfolk Island in the 1990s (Braggins 1996). The species was present in and apparently restricted to the rocky stream banks of Broken Bridge Creek and its tributaries.
Mills (2012b) found the species in 15 colonies in three valleys where it was growing on moist rocks, usually with other small ferns, particularly the large filmy fern Cephalomanes bauerianum. An earlier survey located 36 colonies during a search of the majority of valleys in Norfolk Island National Park (Mills 2007).
In Queensland, the species has been recorded at Kauri Creek, Tinaroo Hills, and Maalan State Forest, Atherton Tableland (Bostock & Spokes 1998).
The distribution within the Norfolk Island Group is shown in Map 57.
Ecology
Epiphytic (grows on, but is not parasitic on, other plants), lithophytic (grows on rocks and cliffs) or terrestrial (grows on the ground) in habit.
Habitat
Grows in moist, humid, shaded forest valleys, often beside waterfalls.
Threats
Threats to the species include drought/dry conditions due to climate change, changes to stream hydrology in the national park, and weed invasion and competition.
Impact on other species
Can grow on branches of other plants.
Map 57 Distribution of Polyphlebium endlicherianum
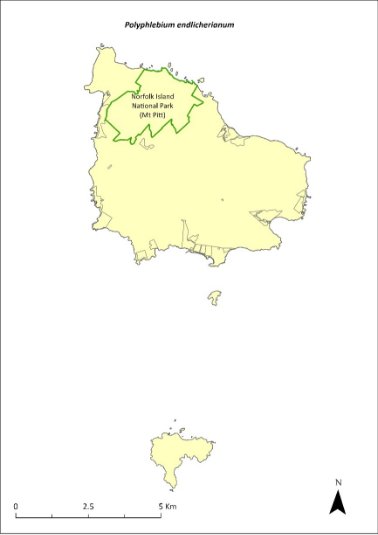
Green outlines indicate reserves within which the species occurs.
Risk assessment
Risk assessment undertaken for Endangered ferns as a grouping. The risk assessment is shown in Table 131.
Table 131 Risk assessment for Endangered ferns as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Rare (0–10%) | Negligible | Negligible |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Possible (26–50%) | Minor | Low |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Likely (51–90%) | Major | High |
15. Problems caused by small populations, including lack of genetic diversity | Likely (51–90%) | Moderate | Medium |
Management actions
This species may require development of species-specific conservation actions, including ex situ conservation. Undertake targeted weed control and maintenance around existing plants, ensuring shaded areas are not opened too quickly. Undertake revegetation/habitat restoration.
Actions may need to be undertaken in collaboration with Queensland Government as appropriate.
Recovery target
The recovery target is shown in Table 132.
Table 132 Recovery target for Polyphlebium endlicherianum
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Endangered | 200 | 100% within the national park | 250 |
Relevant literature
Bostock PD & Spokes TM (1998) Hymenophyllaceae, in PM McCarthy (ed), Flora of Australia 48, ABRS/CSIRO, Canberra. pp. 116–148.
Braggins JE (1996) Report on the conservation status of the ferns of Norfolk Island. Unpublished report to the Australian Nature Conservation Agency.
DCCEEW (2024) Conservation Advice for Polyphlebium endlicherianum (middle filmy fern). Department of Climate Change, Energy, the Environment and Water, Canberra.
Mills K (2007e) The Flora of Norfolk Island. 2. Epiphytes and Mistletoes. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Pteris kingiana—King’s brakefern
Family PTERIDACEAE
Conservation significance
Endemic to Norfolk and Phillip Islands.
EPBC Act Listing Status: Endangered.
Description
A tufted fern with a short erect rhizome and fronds growing to 90 cm long.
Distribution and abundance
Pteris kingiana has been collected from Ball Bay (Orchard 1994) and is known from several scattered sites but never with very many individuals. There were few populations in the Norfolk Island National Park (Braggins 1996).
There were fewer than 200 mature plants recorded in 2003 (TSSC 2003c). Mills (2012b) found 93 plants at two sites within the national park. These populations contained many small plants as well as tall, mature specimens.
Surveys of the public reserves in 2017 (Mills 2017a, b, d, e, f) recorded 7 plants in Point Ross Reserve, 6 plants in Bumbora Reserve, 161 in Cascade Reserve, 45 in Anson Bay Reserve, 28 in Selwyn Reserve, 50 in Hundred Acres Reserve, and 89 in Ball Bay Reserve. The species also occurs on Phillip Island (Mills 2009b).
The population estimate in 2021 was 483.
The distribution is shown in Map 58.
Ecology
Wind dispersed spores. This species grows on shady forest floors, almost always near the coast.
Habitat
The species mainly grows in sheltered coastal forest (Invasive Species Council & TierraMar 2021).
Threats
Threats to the species includes weed invasion and competition, cattle grazing, and clearing of woody weeds without replacing vegetation.
Impact on other species
None known.
Map 58 Distribution of Pteris kingiana
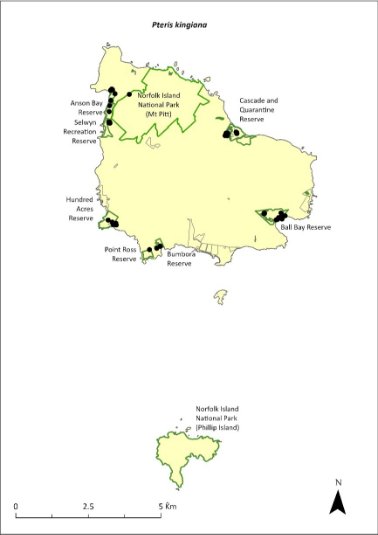
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021). Points show recorded locations (Mills 20017a, b, d, e and f).
Risk assessment
Risk assessment undertaken for Endangered ferns as a grouping. The risk assessment is shown in Table 133.
Table 133 Risk assessment for Endangered ferns as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Rare (0–10%) | Negligible | Negligible |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Moderate | Medium |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Possible (26–50%) | Moderate | Medium |
Management actions
Undertake propagation and replanting into suitable habitat areas. Undertake targeted weed control and maintenance. Implement habitat protection and rehabilitation. Exclude or manage cattle grazing.
Recovery target
The recovery target is shown in Table 134.
Table 134 Recovery target for Pteris kingiana
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Endangered | 483 | 19% within the national park 81% within public reserves | 500 |
Relevant literature
Braggins JE (1996) Report on the conservation status of the ferns of Norfolk Island. Unpublished report to the Australian Nature Conservation Agency.
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
de Lange PJ & Murray BG (2003) Chromosome numbers of Norfolk Island endemic plants. Australian Journal of Botany 51, 211–215.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Mills K (2009b) The Vegetation of Phillip Island, Norfolk Island Group. Envirofund 2007/2008. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2017a) Survey of public reserves on Norfolk Island for threatened plant species: 3. Point Ross Reserve and Bumbora Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017b) Survey of public reserves on Norfolk Island for threatened plant species: 8. Ball Bay Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017d) Survey of public reserves on Norfolk Island for threatened plant species: 6. Anson Bay Reserve and Selwyn Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017e) Survey of public reserves on Norfolk Island for threatened plant species: 7. Hundred Acres Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017f) Survey of public reserves on Norfolk Island for threatened plant species: 4. Cascade Reserve including Quarantine Reserve. Prepared for Norfolk Island Regional Council.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
TSSC (Threatened Species Scientific Committee) (2003c) Commonwealth Listing Advice for Norfolk Island Flora - 16 Endangered Species.
Pteris zahlbruckneriana—netted brakefern
Family PTERIDACEAE
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Endangered.
Description
Tufted fern with a short erect rhizome and fronds growing to 1 m high.
Distribution and abundance
Pteris zahlbruckneriana occurs in scattered populations in forests at higher elevations in the Norfolk Island National Park.
There were fewer than 200 mature plants recorded in 2003 (TSSC 2003c). Mills (2012b) found 35 individuals across seven valleys of the national park, and noted that this species replaces P. kingiana in the upper parts of the valleys leading to the coast. The species is not common and is known to be less widespread than P. kingiana (de Lange & Murray 2003).
The distribution is shown in Map 59.
Ecology
Wind born spores.
Habitat
This species grows in forest on gully sides and creek banks.
Threats
Threats to the species include weed invasion and competition (particularly by William Taylor [Ageratina riparia] and red guava [Psidium cattleyanum]), climate change, and cattle grazing.
Impact on other species
None known.
Map 59 Distribution of Pteris zahlbruckneriana
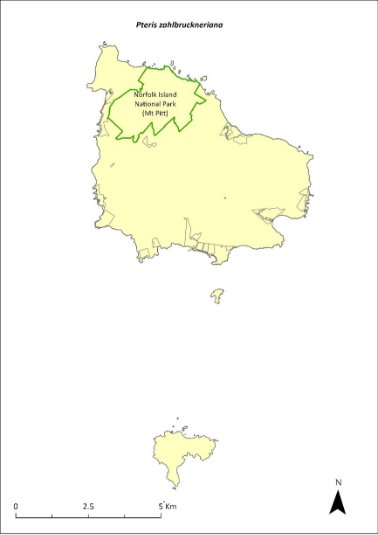
Green outlines indicate reserves within which the species occurs.
Risk assessment
Risk assessment undertaken for Endangered ferns as a grouping. The risk assessment is shown in Table 135.
Table 135 Risk assessment for Endangered ferns as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Rare (0–10%) | Negligible | Negligible |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Moderate | Medium |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Likely (51–90%) | Moderate | Medium |
Management actions
Undertake propagation and replanting into suitable shaded habitat areas. Implement targeted weed control and maintenance. Implement habitat protection and rehabilitation. Exclude or manage cattle grazing.
Recovery target
The recovery target is shown in Table 136.
Table 136 Recovery target for Pteris zahlbruckneriana
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Endangered | 35 | 100% within the national park | 250 |
Relevant literature
Braggins JE (1996) Report on the conservation status of the ferns of Norfolk Island. Unpublished report to the Australian Nature Conservation Agency.
de Lange PJ & Murray BG (2003) Chromosome numbers of Norfolk Island endemic plants. Australian Journal of Botany 51, 211–215.
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
TSSC (Threatened Species Scientific Committee) (2003c) Commonwealth Listing Advice for Norfolk Island Flora – 16 Endangered Species.
Senecio australis—a daisy
Family ASTERACEAE
Conservation significance
Endemic to the Norfolk Island Group. It has recently arrived in New Zealand, where it is known from three small populations comprising a total of 10 or so plants.
EPBC Act Listing Status: Vulnerable.
Description
An erect annual or short-lived perennial growing to 90 cm tall with yellow daisy flowers.
Distribution and abundance
Senecio australis has been recorded from Barney Duffy, Anson Bay, at The Chord at Duncombe Bay, and from the Stool, Phillip Island (Orchard 1994). There were fewer than 500 mature plants recorded in 2003 (TSSC 2003b).
The species was common around the edges of Phillip Island in September 2008, particularly on the southern cliffs (Mills 2009b). It also occurs on Nepean Island (Mills 2009a). It is quite common in some of the public reserves.
The population estimate in 2021 was 1454, including populations in Two Chimneys Reserve (497; Mills 2017g), Anson and Selwyn Reserves (333; Mills 2017d), Cascade Reserve (67; Mills 2017f), Ball Bay Reserve (64; Mills 2017b), Bumbora Reserve (31; Mills 2017a) and Hundred Acres Reserve (26; Mills 2017e).
The distribution is shown in Map 60.
Ecology
Little known.
Habitat
Occurs within coastal pine and white oak forest, coastal white oak shrubland and coastal grassland (Invasive Species Council & TierraMar 2021).
Threats
The primary threat to the species is weed invasion and competition, particularly by kikuyu (Cenchrus clandestinus).
Impact on other species
None known.
Map 60 Distribution of Senecio australis
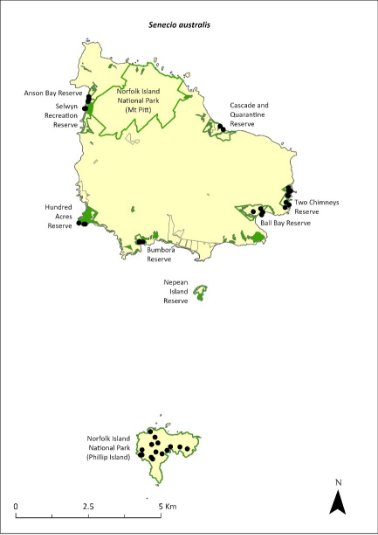
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021, Mills 2009a). Points show recorded locations (Mills 2009b, 2017a, b, d, e, f and g).
Risk assessment
Risk assessment undertaken for Vulnerable herbs/grasses as a grouping. The risk assessment is shown in Table 137.
Table 137 Risk assessment for Vulnerable herbs/grasses as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Rare (0–10%) | Negligible | Negligible |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Moderate | Medium |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Possible (26–50%) | Moderate | Medium |
Management actions
Undertake propagation and replanting into suitable habitat areas. Conduct targeted weed control and maintenance. Implement habitat protection and rehabilitation.
Recovery target
The recovery target is shown in Table 138.
Table 138 Recovery target for Senecio australis
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Vulnerable | 1454 | 34% within the national park 66% within public reserves | 3000 |
Relevant literature
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Mills K (2009a) The Flora of Norfolk Island. 9. The Vegetation of Nepean Island (including Errata and Addenda for Papers 1 to 8). Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2009b) The Vegetation of Phillip Island, Norfolk Island Group. Envirofund 2007/2008. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2017a) Survey of public reserves on Norfolk Island for threatened plant species: 3. Point Ross Reserve and Bumbora Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017b) Survey of public reserves on Norfolk Island for threatened plant species: 8. Ball Bay Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017d) Survey of public reserves on Norfolk Island for threatened plant species: 6. Anson Bay Reserve and Selwyn Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017e) Survey of public reserves on Norfolk Island for threatened plant species: 7. Hundred Acres Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017f) Survey of public reserves on Norfolk Island for threatened plant species: 4. Cascade Reserve including Quarantine Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017g) Survey of public reserves on Norfolk Island for threatened plant species: 5. Two Chimneys Reserve. Prepared for Norfolk Island Regional Council.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
TSSC (Threatened Species Scientific Committee) (2003b) Commonwealth Listing Advice for Norfolk Island Flora - 15 Vulnerable Species.
Senecio evansianus—a daisy
Family ASTERACEAE
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Endangered.
Description
A low herb growing between 3 cm and 30 cm tall with small yellow daisy flowers.
Distribution and abundance
Senecio evansianus has been recorded from Rocky Point, Bumbora Reserve above Creswell Bay, Bloody Bridge and east of Bloody Bridge (Orchard 1994).
There were fewer than 200 mature plants recorded in 2003 (TSSC 2003c). The species has not been found within the Norfolk Island National Park (Mills 2012b) and was not recorded during the 2017 surveys of the public reserves (Mills 2017a-g).
A distribution map is unavailable for Senecio evansianus as there is no reliable information on the current distribution of this species.
Ecology
Little known.
Habitat
The species appears to be restricted to well-watered clay soils beneath open stands of Norfolk Island pine (Araucaria heterophylla; Orchard 1994).
Threats
Primary threats to the species include weed invasion and competition, particularly by kikuyu (Cenchrus clandestinus), and climate change.
Impact on other species
None known.
Risk assessment
The risk assessment is shown in Table 139.
Table 139 Risk assessment for Senecio evansianus
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Rare (0–10%) | Negligible | Negligible |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Major | High |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Major | High |
15. Problems caused by small populations, including lack of genetic diversity | Almost certain (91–100%) | Extreme | Extreme |
Management actions
Conduct surveys to determine current distribution. Undertake propagation and replanting into suitable habitat areas. Conduct targeted weed control and maintenance. Implement habitat protection and rehabilitation. Monitor populations to determine dynamics.
Recovery target
The recovery target is shown in Table 140.
Table 140 Recovery target for Senecio evansianus
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Endangered | 200 | Unknown | 250 |
Relevant literature
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Mills K (2017a) Survey of public reserves on Norfolk Island for threatened plant species: 3. Point Ross Reserve and Bumbora Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017b) Survey of public reserves on Norfolk Island for threatened plant species: 8. Ball Bay Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017c) Survey of public reserves on Norfolk Island for threatened plant species: 1. The Kingston Reserves. Prepared for Norfolk Island Regional Council.
Mills K (2017d) Survey of public reserves on Norfolk Island for threatened plant species: 6. Anson Bay Reserve and Selwyn Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017e) Survey of public reserves on Norfolk Island for threatened plant species: 7. Hundred Acres Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017f) Survey of public reserves on Norfolk Island for threatened plant species: 4. Cascade Reserve including Quarantine Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017g) Survey of public reserves on Norfolk Island for threatened plant species: 5. Two Chimneys Reserve. Prepared for Norfolk Island Regional Council.
TSSC (Threatened Species Scientific Committee) (2003c) Commonwealth Listing Advice for Norfolk Island Flora – 16 Endangered Species.
Senecio hooglandii—a daisy
Family ASTERACEAE
Conservation significance
Endemic to Norfolk Island Group.
EPBC Act Listing Status: Vulnerable.
Description
An erect herb growing to 60 cm tall with yellow daisy flowers.
Distribution and abundance
Senecio hooglandii has been recorded from near the cemetery on Norfolk Island and on the north side of Phillip Island, though its occurrence on Phillip Island may be due to the widespread broadcasting of seed to revegetate the island following rabbit removal (Orchard 1994).
There were fewer than 550 mature plants recorded in 2003 (TSSC 2003b). The species is moderately common around the cliffs of Phillip Island (Mills 2009b) and also occurs on Nepean Island (Mills 2009a).
The distribution is shown in Map 61.
Ecology
Little known.
Habitat
Little known.
Threats
The primary threat to the species is weed invasion and competition, particularly by kikuyu (Cenchrus clandestinus).
Impact on other species
None known.
Map 61 Distribution of Senecio hooglandii
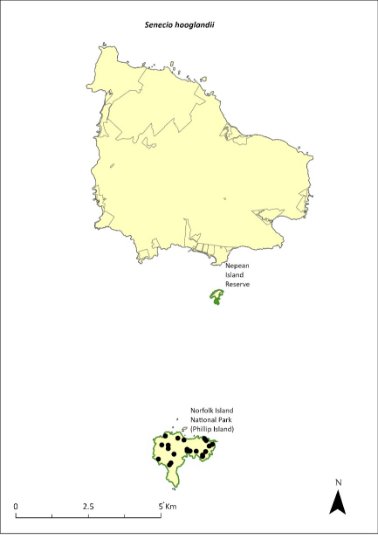
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Mills 2009a). Points show recorded locations (Mills 2009b).
Risk assessment
Risk assessment undertaken for Vulnerable herbs/grasses as a grouping. The risk assessment is shown in Table 141.
Table 141 Risk assessment for Vulnerable herbs/grasses as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Unlikely (11–25%) | Major | Medium |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Almost certain (91–100%) | Moderate | High |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Likely (51–90%) | Moderate | Medium |
Management actions
Undertake propagation and replanting into suitable habitat areas. Conduct targeted weed control and maintenance. Implement habitat protection and rehabilitation. Monitor populations to determine dynamics.
Recovery target
The recovery target is shown in Table 142.
Table 142 Recovery target for Senecio hooglandii
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Vulnerable | 550 | >95% within the national park | 750 |
Relevant literature
Mills K (2009a) The Flora of Norfolk Island. 9. The Vegetation of Nepean Island (including Errata and Addenda for Papers 1 to 8). Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2009b) The Vegetation of Phillip Island, Norfolk Island Group. Envirofund 2007/2008. Kevin Mills & Associates, Jamberoo, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
TSSC (Threatened Species Scientific Committee) (2003b) Commonwealth Listing Advice for Norfolk Island Flora - 15 Vulnerable Species.
Streblus pendulinus—Siah’s backbone
Family MORACEAE
Conservation significance
Streblus pendulinus is endemic to Norfolk Island.
EPBC Act Listing Status: Endangered.
Approved Conservation Advice: 1/07/2016 (TSSC 2016c).
Description
Tree or shrub growing to 6 m tall with fleshy red fruit and very rough leaves. It exudes a white latex when damaged.
Distribution and abundance
In 1988 Streblus pendulinus was not generally common but was widespread and not considered threatened on Norfolk Island (Sykes & Atkinson 1988). The species has been recorded from Cascade Reserve, Mt Pitt, Mt Pitt Road, and about 3km north-east of the cemetery at Kingston (Orchard 1994). It was listed as Endangered in 2003, when most surviving individuals of the species were inside the Norfolk Island National Park (187 mature individuals; TSSC 2003c), Additional plants have been recorded in the Mission Road rainforest remnants, near Steels Point and in Ball Bay Reserve.
Mills (2012b) found the species on 16 transects within the national park, with 107 plants counted. All age classes were observed, and good regeneration was reported. The species also occurs in Cascade, Ball Bay and Selwyn Reserves, in small numbers (Mills 2017b, d and f).
The population estimate in 2021 was 259.
The distribution is shown in Map 62.
Ecology
Many trees are male and cannot produce seed (Sykes & Atkinson 1988), although male and female flowers can be seen on individual trees (K Mills 2024. pers comm 11 January).
Habitat
Occurs in sheltered coastal forest (Invasive Species Council & TierraMar 2021).
Threats
The main threats to the species are competition from weeds and cattle grazing (TSSC 2016c). Phytophthora cinnamomi is potentially a major risk.
At the time of the previous recovery plan (Director of National Parks 2010), a parasite appeared to be stopping seed setting in many individuals (TSSC 2016c).) Insect larvae (possibly a moth species) appear to feed on the developing fruit and destroy the seeds (K Mills 2024. pers comm 11 January). This requires further investigation.
Map 62 Distribution of Streblus pendulinus
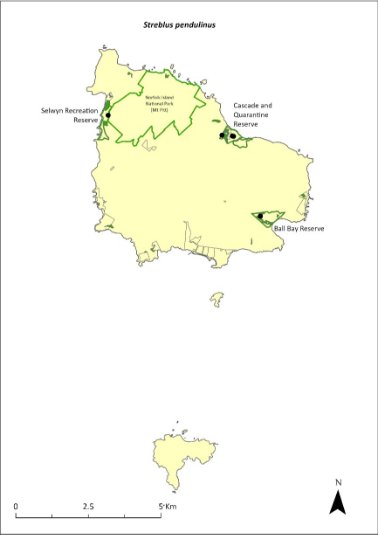
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021). Points show recorded locations (Mills 20017b, d and f).
Impact on other species
None known.
Risk assessment
Risk assessment undertaken for Endangered trees/shrubs as a grouping. The risk assessment is shown in Table 143.
Table 143 Risk assessment for Endangered trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Almost certain (91–100%) | Major | Extreme |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Likely (51–90%) | Moderate | Medium |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Moderate | Medium |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Possible (26–50%) | Major | High |
Management actions
Undertake research to determine the best method of treating the parasite. Implement targeted weed control and maintenance. Implement habitat protection and rehabilitation. Exclude or manage cattle grazing.
Recovery target
The recovery target is shown in Table 144.
Table 144 Recovery target for Streblus pendulinus
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Endangered | 259 | 95% within the national park 5% within public reserves | 1000 |
Relevant literature
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
Director of National Parks (2010) Norfolk Island Region Threatened Species Recovery Plan. Department of the Environment, Water, Heritage and the Arts, Canberra.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2017b) Survey of public reserves on Norfolk Island for threatened plant species: 8. Ball Bay Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017d) Survey of public reserves on Norfolk Island for threatened plant species: 6. Anson Bay Reserve and Selwyn Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017f) Survey of public reserves on Norfolk Island for threatened plant species: 4. Cascade Reserve including Quarantine Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2024) Personal communication by email, 11 January 2024, plant ecologist.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003c) Commonwealth Listing Advice for Norfolk Island Flora – 16 Endangered Species.
Threatened Species Scientific Committee (TSCC) (2016c) Conservation Advice Streblus pendulinus Siah’s backbone. Department of the Environment, Canberra.
Taeniophyllum norfolkianum—minute orchid, ribbon‑root orchid
Family ORCHIDACEAE
Conservation significance
Taeniophyllum norfolkianum was considered endemic at listing; it has now also been reported from New Zealand (Renner & Beadel 2011).
EPBC Act Listing Status: Vulnerable.
Description
A small epiphytic orchid with tiny greenish yellow flowers.
Distribution and abundance
T. norfolkianum has been recorded from Mt Bates (Orchard 1994), south of Mount Pitt and in the vicinity of Red Road (K Mills 2024. pers comm 11 January). There were fewer than 500 mature plants recorded in 2003 (TSSC 2003b).
The distribution is shown in Map 63.
Ecology
Leafless with photosynthetic roots.
Habitat
Grows on the trunks and underside of branches of the Norfolk Island pine (Araucaria heterophylla).
Threats
The species is threatened by small population size and subsequent increased risk of extinction through natural events such as cyclones, slips and drought, and by climate change.
Impact on other species
Primarily grows on Norfolk Island pines.
Map 63 Distribution of Taeniophyllum norfolkianum
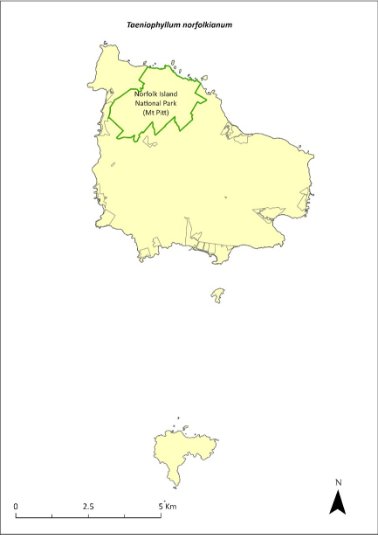
Green outlines indicate reserves within which the species occurs.
Risk assessment
Risk assessment undertaken for all threatened orchids as a grouping. The risk assessment is shown in Table 145.
Table 145 Risk assessment for all threatened orchids as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Minor | Medium |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Moderate | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Minor | Medium |
4. Degradation of native vegetation through current or future grazing | Unlikely (11–25%) | Moderate | Low |
6. Predation by rodents | Rare (0–10%) | Negligible | Negligible |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Possible (26–50%) | Moderate | Medium |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Moderate | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Likely (51–90%) | Major | High |
15. Problems caused by small populations, including lack of genetic diversity | Likely (51–90%) | Moderate | Medium |
Management actions
This orchid may require development of species-specific conservation actions, including ex situ conservation. Implement targeted weed control and maintenance. Implement habitat protection and rehabilitation. Undertake research into the ecology of the species. Monitor/survey likely areas of the national park after storms, rescue any fallen specimens and attempt to cultivate them in at the Norfolk Island National Park Nursery (Sykes & Atkinson 1988).
Recovery target
The recovery target is shown in Table 146.
Table 146 Recovery target for Taeniophyllum norfolkianum
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Vulnerable | 500 | 100% within the national park | No decline |
Relevant literature
Mills K (2024) Personal communication by email, 11 January 2024, plant ecologist.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Renner MAM & Beadel SM (2011) Taeniophyllum norfolkianum: a second genus of Vandeae (Orchidaceae) indigenous to New Zealand. New Zealand Journal of Botany 49(3), 435-439.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003b) Commonwealth Listing Advice for Norfolk Island Flora - 15 Vulnerable Species.
Tmesipteris norfolkensis—hanging fork-fern
Family PSILOTACEAE
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Vulnerable.
Description
A small, pendulous epiphytic fern with branches growing to 25 cm long.
Distribution and abundance
Tmesipteris norfolkensis has been recorded on the south-east slopes of Mount Pitt and between Palm Glen and Red Road (Orchard 1994). It appears to be restricted to moist forests and is most common on the southern side of the mountains rather than the drier northern side (Mills 2007e).
There were fewer than 500 mature plants recorded in 2003 (TSSC 2003b), with most occurring in damp and shady valleys of the Mt Pitt section of the Norfolk Island National Park, where it grows on the lower part of tree fern trunks (Sykes & Atkinson 1988).
The distribution is shown in Map 64.
Ecology
An epiphyte that grows in damp conditions and uses several native hardwoods as hosts but prefers the fibrous base of the tree ferns Sphaeropteris excelsa (under 1 m from the ground) and Alsophila australis norfolkensis.
Most plants grow on the downhill side of the trunks of the tree ferns (Mills 2007e).
Habitat
Grows in damp and shady places and prefers the deep moist valleys of the southern side of the mountains.
Threats
Threats to the species include habitat degradation, catastrophic events such as severe storms, and climate change.
Impact on other species
Grows on tree ferns and on several hardwood tree hosts.
Map 64 Distribution of Tmesipteris norfolkensis
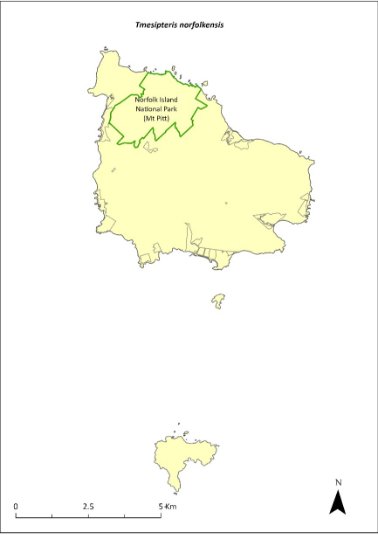
Green outlines indicate reserves within which the species occurs.
Risk assessment
Risk assessment undertaken for Vulnerable ferns as a grouping. The risk assessment is shown in Table 147.
Table 147 Risk assessment for Vulnerable ferns as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Rare (0–10%) | Negligible | Negligible |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Unlikely (11–25%) | Moderate | Low |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Possible (26–50%) | Moderate | Medium |
Management action
Implement targeted weed control and maintenance around host trees. Implement habitat protection and rehabilitation. Monitor/survey likely areas of the national park after storms, rescue any fallen specimens and attempt to cultivate them in at the Norfolk Island National Park Nursery (Sykes & Atkinson 1988).
Recovery target
The recovery target is shown in Table 148.
Table 148 Recovery target for Tmesipteris norfolkensis
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Vulnerable | 500 | 100% within the national park | 1000 |
Relevant literature
Mills K (2007e) The Flora of Norfolk Island. 2. Epiphytes and Mistletoes. Kevin Mills & Associates, Jamberoo, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003b) Commonwealth Listing Advice for Norfolk Island Flora - 15 Vulnerable Species.
Ungeria floribunda—bastard oak
Family STERCULIACEAE
Conservation significance
Endemic monotypic genus which could be one of Norfolk Island’s most ancient plants.
EPBC Act Listing Status: Vulnerable.
Description
A tree growing to 15 m tall with deep pink flowers.
Distribution and abundance
Ungeria floribunda is evenly distributed through the Norfolk Island National Park. It occurs on the broad ridges and upper valley sides and is also found on flat sites outside the park in the north-west part of the island. Young trees are associated with secondary forest that has established following removal of the original canopy.
The total number of mature plants recorded in 2003 was 502 (TSSC 2003b).
Regeneration is restricted by predation of seeds by rats (either on the tree or on the ground) and because this species is a periodic regenerator that does not fruit every year (Sykes & Atkinson 1988). It may require 20 years or more to produce viable seed.
U. floribunda propagates well from seed. The species seems to be regenerating well, mainly occurring above 120 metres altitude, although seedling survival appears to be low (K Mills 2024. pers comm 11 January).
The distribution is shown in Map 65.
Ecology
Little known.
Habitat
This species grows in forested areas throughout Norfolk Island, especially in areas of dense canopy above moderate elevations (such as Prince Phillip Drive/Red Road area, including private land; M Christian 2024. pers comm 12 January). Occurs in moist upland hardwood forest and pine-hardwood ridge forest (Invasive Species Council & TierraMar 2021).
Threats
Threats to the species include seed predation by rats, and irregular seed production. Phytophthora cinnamomi is potentially a major risk.
Impact on other species
None known.
Map 65 Distribution of Ungeria floribunda
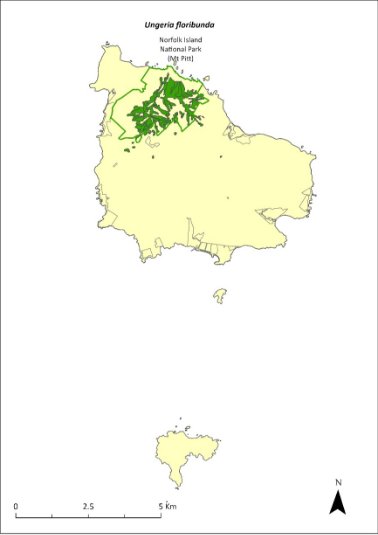
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021).
Risk assessment
Risk assessment undertaken for Vulnerable trees/shrubs as a grouping. The risk assessment is shown in Table 149.
Table 149 Risk assessment for Vulnerable trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Almost certain (91–100%) | Extreme | Extreme |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Moderate | Medium |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Possible (26–50%) | Moderate | Medium |
Management actions
Undertake habitat protection and rehabilitation. Undertake seed propagation (when seed is available) and replanting in suitable habitat. Carry out rodent control. Carry out targeted weed control and maintenance around existing populations.
Recovery target
The recovery target is shown in Table 150.
Table 150 Recovery target for Ungeria floribunda
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Vulnerable | 502 | >95% within the national park | 1000 |
Relevant literature
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
Christian M (2024) Personal communication by email, 12 January 2024, independent researcher.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Mills K (2024) Personal communication by email, 11 January 2024, plant ecologist.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003b) Commonwealth Listing Advice for Norfolk Island Flora - 15 Vulnerable Species.
Wikstroemia australis—kurrajong
Family THYMELAEACEAE
Conservation significance
Endemic to Norfolk Island.
EPBC Act Listing Status: Critically Endangered.
Description
A small tree growing to 4 m or taller, with blackish stems and hard, rough bark.
Distribution and abundance
Wikstroemia australis was once widespread over much of Norfolk Island, but since the 1930s it has been reduced to scattered pockets mostly within the Norfolk Island National Park (Tierney 1989).
In 1988, it was widely distributed on the ridges and upper valley sides of the Mt Pitt section of the national park, but there was a critical lack of juvenile plants (Sykes & Atkinson 1988). The lack of regeneration of young plants was probably caused by competition from weeds, particularly red guava (Psidium cattleyanum), unsuitable soil conditions, dry conditions due to climate change and disease (Sykes & Atkinson 1988).
Tierney (1989) noted that young plants successfully established in gaps along the Bird Rock Track with young plants relatively common close to the track in less weedy areas. However, a survey in 1989 suggested a continued decline, with many diseased plants (Gilmour & Helman 1989b).
The total number of mature plants recorded in 2003 was 155 (TSSC 2003g).
A targeted survey by Mills (2010) found a total of 84 plants, including trees and a reasonable number of seedling. Mills (2012b) counted 12 plants at five locations, but noted that the species was not regenerating well, as most seedlings were not surviving.
The population estimate in 2021 had increased to 629 individuals, through planting and propagation as part of the Norfolk Island National Park threatened flora program.
The distribution is shown in Map 66.
Ecology
This species is possibly short lived and requires high light levels for establishment.
Habitat
Occurs in moist upland hardwood forest and pine-hardwood ridge forest (Invasive Species Council & TierraMar 2021), especially in protected, sunny, moist sites.
Threats
Threats to the species include weed invasion and competition from weeds, particularly red guava. Phytophthora cinnamomi is potentially a major risk. There has been unexplained death of many plants; the cause of this is unknown.
Map 66 Distribution of Wikstroemia australis
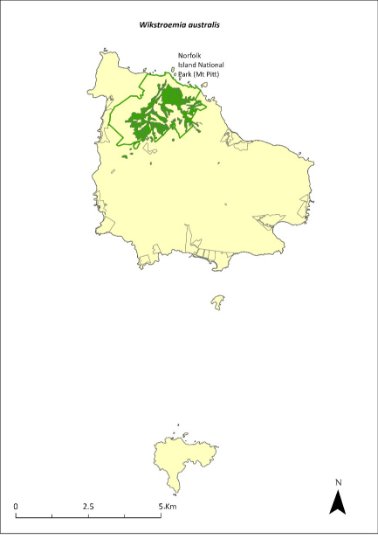
Green outlines indicate reserves within which the species occurs. Green shading shows plant communities within which the species may occur (Christian & Mills 2021).
Impact on other species
None known.
Risk assessment
Risk assessment undertaken for Critically Endangered trees/shrubs as a grouping. The risk assessment is shown in Table 151.
Table 151 Risk assessment for Critically Endangered trees/shrubs as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Almost certain (91–100%) | Major | Extreme |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Likely (51–90%) | Moderate | Medium |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Possible (26–50%) | Minor | Low |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Minor | Medium |
12. Infection by pathogens already present | Possible (26–50%) | Moderate | Medium |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Likely (51–90%) | Moderate | Medium |
Management actions
Continue propagation and planting in suitable areas. Undertake habitat protection and rehabilitation. Carry out targeted weed control and maintenance around existing populations to create gaps to allow the penetration of sunlight. Undertake research into the causes of plant death and treatment options.
Recovery target
The recovery target is shown in Table 152.
Table 152 Recovery target for Wikstroemia australis
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Critically Endangered | 629 | >95% within the national park | 1000 |
Relevant literature
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
Gilmour PM & Helman CE (1989b) The Vegetation of Norfolk Island National Park. Report to the Australian National Parks and Wildlife Service, Norfolk Island.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Mills K (2010) The Flora of Norfolk Island. 11. Field Survey and Assessment of the Critically Endangered Endemic Plant Wikstroemia australis (Kurrajong). Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003g) Listing Advice—Critically Endangered Wikstroemia australis (Kurrajong).
Tierney JW (1989) Report on investigation into kurrajong (Wikstroemia australis) decline and Phellinus noxius root rot control on Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
Zehneria baueriana—native cucumber, giant cucumber
Family CUCURBITACEAE
Conservation significance
In Australia it is restricted to Norfolk Island, but also occurs in New Caledonia.
EPBC Act Listing Status: Endangered.
Description
A large perennial climber with corky rope-like stems and red, fleshy berries.
Distribution and abundance
Zehneria baueriana has been collected from Mt Pitt (Orchard 1994) and recorded in the Mt Pitt and Phillip Island sections of the Norfolk Island National Park and in the Mission Road rainforest remnants. On Phillip Island, it occurs mainly in the highest parts of the Long Valley catchment (Mills 2009b).
There were 77 mature individuals recorded in 2003 (TSSC 2003c). By 2010, the Mission Road rainforest remnants contained the largest clumps of this species, and it was scattered throughout the national park as individuals (Director of National Parks 2010).
Mills (2012b) found the species to be quite common throughout the national park. 180 plants were counted during 2012 making it one of the most common species seen during flora surveys. The survey found a range of plant age classes from small seedlings to plants climbing high into trees, and the species occurred on nearly all transects.
The distribution is shown in Map 67.
Ecology
Occurs in light gaps and is rather transient in its occurrence. Little else is known about the ecology of the species.
Habitat
This species is a locally common climber that primarily grows in gaps in the forest and around the edges, climbing high into trees (Mills 2012b).
Threats
Threats to the species include weed invasion and competition, cattle grazing, and predation of fruit by rodents.
Impact on other species
Climbs on other vegetation.
Map 67 Distribution of Zehneria baueriana
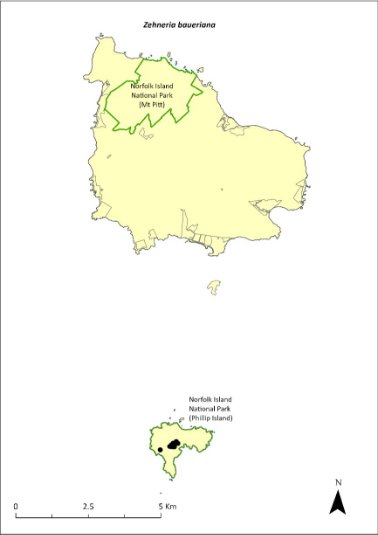
Green outlines indicate reserves within which the species occurs. Points show recorded locations (Mills 2009b).
Risk assessment
Risk assessment undertaken for Endangered vines/climbers as a grouping. The risk assessment is shown in Table 153.
Table 153 Risk assessment for Endangered vines/climbers as a grouping
Risk | Likelihood of exposure | Consequence | Threat rating |
1. Loss and fragmentation of native vegetation through past land clearing | Almost certain (91–100%) | Extreme | Extreme |
2. Loss and fragmentation of native vegetation through current or future land clearing | Rare (0–10%) | Negligible | Negligible |
3. Degradation of native vegetation through past grazing or loss of nutrients | Almost certain (91–100%) | Extreme | Extreme |
4. Degradation of native vegetation through current or future grazing | Possible (26–50%) | Moderate | Medium |
6. Predation by rodents | Almost certain (91–100%) | Negligible | Negligible |
7. Predation by cats | Rare (0–10%) | Negligible | Negligible |
8. Predation or damage by chickens | Rare (0–10%) | Negligible | Negligible |
9. Predation by swamphens | Rare (0–10%) | Negligible | Negligible |
10. Predation by Argentine ant | Rare (0–10%) | Negligible | Negligible |
11. Competition from/change of habitat because of weed invasion | Likely (51–90%) | Moderate | Medium |
12. Infection by pathogens already present | Rare (0–10%) | Negligible | Negligible |
13. Impacts of potential new invasive species or pathogens | Unlikely (11–25%) | Minor | Low |
14. Changes to vegetation, increased fire risk and/or direct physiological stress as a result of climatic changes | Possible (26–50%) | Moderate | Medium |
15. Problems caused by small populations, including lack of genetic diversity | Possible (26–50%) | Moderate | Medium |
Management actions
Conduct targeted weed control and maintenance around existing populations. Implement habitat protection and rehabilitation. Undertake seed propagation (when seed is available) and replanting in suitable habitat. Exclude or manage cattle grazing.
Recovery target
The recovery target is shown in Table 154.
Table 154 Recovery target for Zehneria baueriana
EPBC Act status | Estimated population (2023) | Where known populations occur | 2034 target |
Endangered | 180 groups of plants | >95% within the national park | 300 groups of plants |
Relevant literature
Director of National Parks (2010) Norfolk Island Region Threatened Species Recovery Plan. Department of the Environment, Water, Heritage and the Arts, Canberra.
Mills K (2009b) The Vegetation of Phillip Island, Norfolk Island Group. Envirofund 2007/2008. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Norfolk Island.
TSSC (Threatened Species Scientific Committee) (2003c) Commonwealth Listing Advice for Norfolk Island Flora - 16 Endangered Species
Term | Definition |
Australian Government | The Government of the Commonwealth of Australia |
APC | The Australian Plant Census |
AFD | The Australian Faunal Directory |
CHL | Commonwealth Heritage List |
DCCEEW | The Department of Climate Change, Energy, the Environment and Water including any such other department or agency that succeeds to the functions of the Department |
DITRDCA | The Department of Infrastructure, Transport, Regional Development, Communications and the Arts including any such other department or agency that succeeds to the functions of the Department |
The Director of National Parks (or Director) | The Director is a corporation-sole under the EPBC Act and a corporate Commonwealth entity for the purposes of the Public Governance, Performance and Accountability Act 2013. The corporation is constituted by the person appointed by the Governor-General to the office that is also called the Director of National Parks. The functions of the Director include the administration, management and control of the Norfolk Island National Park and Botanic Garden. The Director of National Parks includes any person to whom the Director has delegated powers and functions under the EPBC Act. The Director is supported by Parks Australia, a division of the Department of Climate Change, Energy, the Environment and Water (DCCEEW). |
EPBC Act | The Environment Protection and Biodiversity Conservation Act 1999 including Regulations under the Act, and includes reference to any Act amending, repealing or replacing the EPBC Act |
EPBC Regulations | The Environment Protection and Biodiversity Conservation Regulations 2000 and includes reference to any Regulations amending, repealing or replacing the EPBC Regulations |
IUCN | The International Union for Conservation of Nature |
KAVHA | Kingston and Arthur’s Vale Historic Area |
Key threatening process | A process that threatens or may threaten the survival, abundance or evolutionary development of a native species or ecological community, as identified and listed under the EPBC Act |
Minister | The Minister administering the EPBC Act |
MNES | A Matter of National Environmental Significance are matters that are protected under national environmental law and include, for example listed threatened species and communities under the EPBC Act |
NINPAC | The Norfolk Island National Park Advisory Committee which is responsible for advising the Park Manager on implementation of the Norfolk Island National Park management plan |
NIRC | The Norfolk Island Regional Council which is responsible for local government functions on Norfolk Island and may deliver some other including state-type functions under agreements with DITRDCA |
Norfolk Island Botanic Garden or botanic garden | The area declared as a reserve by that name under the National Parks and Wildlife Conservation Act 1975 and continued under the EPBC Act by the Environmental Reform (Consequential Provisions) Act 1999 |
Norfolk Island Group | The group of islands that are included in the plan including Norfolk Island, Phillip Island, Nepean Island, and the surrounding rock stacks |
Norfolk Island National Park | The areas declared as a national park by that name under the NPWC Act and continued under the EPBC Act by the Environmental Reform (Consequential Provisions) Act 1999 |
NPWC Act | The National Parks and Wildlife Conservation Act 1975 and the Regulations under that Act |
Park or national park | Norfolk Island National Park |
Parks Australia | The part of the Department of Climate Change, Energy, the Environment and Water that assists the Director of National Parks in performing the Director’s functions under the EPBC Act |
Pressure (or threat) | Event, condition or process that results in degradation of the environment | |
Recovery plan or plan | This Norfolk Island Region Threatened Species Recovery Plan, unless otherwise stated |
Territory | The Territory of Norfolk Island |
Threat abatement plan | A statutory document aimed at lessening the impact of a key threatening process, as identified and listed under the EPBC Act |
Threatened species | A species listed as Vulnerable, Endangered or Critically Endangered under the EPBC Act |
Threatening process | A process or activity that ‘threatens … the survival, abundance or evolutionary development of a native species or ecological community’ (EPBC Act, p. 273) and which also may threaten the sustainability of resource use |
Risk | The combined likelihood of exposure to an environmental pressure and the level of harmful consequences |
| | | |
Abell RS & Falkland AC (1991) The hydrogeology of Norfolk Island, South Pacific Ocean (Bulletin‑Australia, Bureau of Mineral Resources, Geology and Geophysics, 234). Australian Government Public Service, Canberra.
Abell RS & Taylor FJ (1981). Hydrogeological and geophysical investigations on Norfolk Island. Geoscience Australia, Canberra.
Anderson AJ (1996) Discovery of a prehistoric habitation site on Norfolk Island. Journal of the Polynesian Society 105, 479–486.
Anderson AJ, Smith I & White P, 2001. Archaeological fieldwork on Norfolk Island. Records of the Australian Museum, Supplement 27, 11–32.
Anderson JG & Cochrane K (1995) Assessment of Population Numbers of Norfolk’s Threatened Plants (Norfolk Island National Park). Report to the Australian Nature Conservation Agency, Norfolk Island.
Australian Biological Resources Study (ABRS) (2017) Lichens of Norfolk Island, South Pacific Ocean. Accessed 30 January 2024.
Australian Museum (2022) Priceless archaeological artefacts found in Norfolk Island National Park by local citizen scientist. Accessed 31 January 2024.
Bell BD (1990) The status and management of the White-breasted White-eye and other birds of Norfolk Island. Unpublished report to the Australian Nature Conservation Agency.
Benson ML (1980) Dieback of Norfolk Island pine in its natural environment. Australian Forestry 43, 245–252.
Bester A (2003) The breeding, foraging ecology and conservation of the Providence Petrel Pterodroma solandri on Lord Howe Island, Australia. PhD Thesis, Charles Sturt University, Albury, NSW.
Bindoff NL, Cheung WWL, Kairo JG, Arístegui J, Guinder VA, Hallberg R, Hilmi N, Jiao N, Karim MS, Levin L, O’Donoghue S, Purca Cuicapusa SR, Rinkevich B, Suga T, Tagliabue A & Williamson P (2019) Changing Ocean, Marine Ecosystems, and Dependent Communities, in H-O Pörtner, DC Roberts, V Masson-Delmotte, P Zhai, M Tignor, E Poloczanska, K Mintenbeck, A Alegrı́a, M Nicolai, A Okem, J Petzold, B Rama & NM Weyer (eds), IPCC Special Report on the Ocean and Cryosphere in a Changing Climate. Cambridge University Press, Cambridge, UK and New York, NY, USA.
Blakers M, Davies SJJF & Reilly PM (1984) An atlas of Australian birds. Royal Australasian Ornithologists Union, Melbourne University Press, Melbourne.
Borges PA, Gabriel R & Fattorini S (2020) Biodiversity erosion: causes and consequences, in Life on Land. Encyclopedia of the UN Sustainable Development Goals. Springer International Publishing, Cham, Switzerland.
Bostock PD & Spokes TM (1998) Hymenophyllaceae, in PM McCarthy (ed), Flora of Australia 48, ABRS/CSIRO, Canberra. pp. 116–148.
Braggins JE (1996) Report on the conservation status of the ferns of Norfolk Island. Unpublished report to the Australian Nature Conservation Agency.
Brown SM, Macgregor N, Wilson M, Olsen P, Clarke R, Lees C, Weeks A, Heinsohn R, Pryde M, Kavanagh R, Greenwood D, Ward R, Latch P, Christian M, Christian J, Greenup N, Lang S, Quintal R & Christian K (2020) Report on an expert workshop to discuss conservation strategies for the Norfolk Island morepork (Ninox novaeseelandiae undulata) and other Norfolk Island birds. Director of National Parks, Canberra.
Brownsey PJ & Chinnock RJ (1987) A taxonomic revision of the Australian species of Hypolepis. Journal of the Adelaide Botanical Garden 10, 1–30.
Bureau of Meteorology (2019) Temperature and rainfall changes at remote Australian Islands and Antarctic sites. Accessed 30 January 2024.
Bureau of Meteorology (2021) Norfolk Island climate. Accessed 30 January 2024.
Cai WJ (2011) Estuarine and coastal ocean carbon paradox: CO2 sinks or sites of terrestrial carbon incineration? Annual Review of Marine Science 3, 123–145.
Carlile N (2011) Observations of seabirds on Phillip Island 8–12 May 2011. Unpublished report.
Carlile N & O’Dwyer T (2018) NI2016–26 Report to the Director national parks and Manager Norfolk Island National Park. Office of Environment and Heritage NSW.
Carlile N & O’Dwyer T (2022) At-sea movements of the White Tern Gygis alba in waters off Eastern Australia. Marine Ornithology 50, 157–164.
Carlile N & O’Dwyer T (2023) Conservation of the surface-nesting Kermadec Petrel Pterodroma neglecta neglecta in the South Pacific: Clarifying breeding ecology and the threat of avian ground predators. Bird Conservation International 33, e44, 1–9.
Carlile N, O'Dwyer T, Wilson M, Clarke RH, Brown SM, Baker GB & Garnett ST (2021) Western Kermadec petrel Pterodroma neglecta neglecta, in ST Garnett & GB Baker (eds), The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne. pp. 169–172.
Carlile N, Priddel D, Lloyd C, Jarman M & Craven P (2015) Seabird islands No. 42 (1): Tollgate Islands, New South Wales. Corella 39(4), 96–99.
Cayzer LW, Crisp MD & Telford IRH (2000) A revision of Pittosporum (Pittosporaceae) in Australia. Systematic Journal of Botany 13, 845–902.
Chapple D, Tingley R, Mitchell N, Macdonald S, Keogh JS, Shea G, Bowles P, Cox N & Woinarski J (2019) The Action Plan for Australian Lizards and Snakes 2017. CSIRO Publishing, Melbourne.
Christian M (2005) Norfolk Island …the birds. Green Eyes Publications, Norfolk Island.
Christian N (1999) A study of the determinants of invasive success and management options for the weed species Psidium cattleianum Sabine var cattleianum (Strawberry guava) in Norfolk Island. Honours thesis, Southern Cross University.
Christian NE & Mills K (2021) Vegetation Mapping of Norfolk Island 2021. Unpublished data.
Clark BL, Carneiro APB, Pearmain EJ et al. (2023) Global assessment of marine plastic exposure risk for oceanic birds. Nature Communications 14, 3665.
Clarke RH, Dutson G, Olsen P & Garnett ST (2021) White-chested White-eye Zosterops albogularis, in ST Garnett & GB Baker (eds), The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne. pp. 762–763.
Cogger HG (2004) Draft recovery plan for the threatened lizards Christinus guentheri and Oligosoma lichenigera on Norfolk and Lord Howe Islands. Unpublished draft report to Department of the Environment and Heritage, Canberra.
Cogger HG, Cameron EE & Sadlier RA (1979) The terrestrial reptiles of islands in the Norfolk Island complex. Unpublished report to the Australian National Parks and Wildlife Service, Canberra.
Cogger HG, Cameron EE, Sadlier RA & Eggler P (1993) The Action Plan for Australian Reptiles. Australian Nature Conservation Agency, Canberra.
Cogger HG, Muir G & Shea G (2006) A survey of the terrestrial reptiles of Norfolk Island March 2005: Reports 1–4. Unpublished reports to the Department of the Environment and Heritage, Canberra.
Cole NC, Jones CG & Harris S (2005) The need for enemy-free space: The impact of an invasive gecko on island endemics. Biological Conservation 125(4), 467–474.
Commonwealth of Australia (2005) National Recovery Plan for the Norfolk Island Scarlet Robin Petroica multicolor multicolor and the Norfolk Island Golden Whistler Pachycephala pectoralis xanthroprocta. Department of the Environment and Heritage, Canberra.
Commonwealth of Australia (2009). Threat abatement plan to reduce the impacts of exotic rodents on biodiversity on Australian offshore islands of less than 100 000 ha. Department of the Environment, Water, Heritage and the Arts, Canberra. Accessed 31 January 2024.
Commonwealth of Australia (2015a) Threat abatement plan for predation by feral cats. Department of the Environment, Canberra. Accessed 31 January 2024.
Commonwealth of Australia (2015b) Wildlife Conservation Plan for Migratory Shorebirds. Department of the Environment, Canberra. Accessed 1 February 2024.
Commonwealth of Australia (2018a) Threat abatement plan for the impacts of marine debris on the vertebrate wildlife of Australia's coasts and oceans. Department of the Environment and Energy, Canberra. Accessed 1 February 2024.
Commonwealth of Australia (2018b) Threat abatement plan for the incidental catch (or bycatch) of seabirds during oceanic longline fishing operations. Department of the Environment and Energy, Canberra. Accessed 1 February 2024.
Commonwealth of Australia (2022) Wildlife Conservation Plan for Seabirds. Accessed 30 January 2024.
Connor HE (1990) Elymus (Gramineae) on Norfolk Island. Kew Bulletin 45, 680.
Cowie RH (2001) Decline and homogenization of Pacific faunas: the land snails of American Samoa. Biological Conservation 99, 207–222.
Coyne P, Evans B, Evans O & McCoy H (2015) The Tasman Masked Booby Sula dactylatra tasmani of Nepean and Phillip Islands in the Norfolk Island Group. Corella 39 (3), 60–66.
CSIRO (2020) Norfolk Island Water Resource Assessment. A summary report from the CSIRO Norfolk Island Water Resource Assessment. CSIRO, Australia.
CSIRO, Managers of World Heritage Properties in Australia & Indigenous Reference Group (2021) The implications of climate change for World Heritage Properties in Australia: assessment of impacts and vulnerabilities. Department of Climate Change, Energy, the Environment and Water, Canberra.
Csurhes S & Markula A (2009) Pest animal risk assessment: Asian house gecko Hemidactylus frenatus. Biosecurity Queensland, Queensland Primary Industries and Fisheries, Department of Employment, Economic Development and Innovation, Queensland.
Dann LE, Guja L, Kark S & Dwyer J (2023) Comparative study reveals management of a dominant invasive plant facilitates subtropical forest regeneration. Biological Invasions 26, 299–313.
Dann LE, Scott M, Guja L, Wilson M, Greenup N & Kark S (2021) A guide to propagating Norfolk Island’s native plants and seeds. National Environmental Science Program Threatened Species Recovery Hub, The University of Queensland, Brisbane.
Davidson P, Anderson J & Evans O (1994) Native vegetation within the forestry zone of Norfolk Island National Park. Unpublished Report to the Australian Nature Conservation Agency.
Davis P (2008) Argentine Ants on Norfolk Island—An investigation into their extent and future management options, Report of a visit 4th–10th May 2008. Unpublished report to Administration of Norfolk Island.
Davidson P (2008) Collection of Blood Samples from Providence Petrel Pterodroma solandri on Phillip Island, Norfolk Island Group 12–14 June 2008. Unpublished report.
Dawkins K & Esiobu N (2016) Emerging insights on Brazilian pepper tree (Schinus terebinthifolius) invasion: the potential role of soil microorganisms. Frontiers in plant science 7, 712.
DCCEEW (Department of Climate Change, Energy, the Environment and Water) (n.d.). Australian Heritage Database. Available on the Internet at: https://www.environment.gov.au/heritage/publications/australian-heritage-database
DCCEEW (Department of Climate Change, Energy, the Environment and Water) (2022a) Nature Positive Plan: better for the environment, better for business. Department of Climate Change, Energy, the Environment and Water, Canberra.
DCCEEW (Department of Climate Change, Energy, the Environment and Water) (2022b) Threatened Species Strategy Action Plan 2022–2032. Department of Climate Change, Energy, the Environment and Water, Canberra.
DCCEEW (Department of Climate Change, Energy, the Environment and Water) (2024) Conservation Advice for Polyphlebium endlicherianum (middle filmy fern). Department of Climate Change, Energy, the Environment and Water, Canberra.
Debus SJS (2012) Norfolk Island Boobook chick deaths. Boobook 30, 6.
DECC (Department of Environment and Climate Change) NSW (2007) Lord Howe Island Biodiversity Management Plan. Department of Environment and Climate Change, NSW, Sydney.
DEH (Department of the Environment and Heritage) (2006) Threat abatement plan to reduce the impacts of tramp ants on biodiversity in Australia and its territories. Department of the Environment and Heritage, Canberra.
DEH (Department of the Environment and Heritage) (2003) What the Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act) means for Norfolk Island. Department of the Environment and Heritage, Canberra.
de Lange PJ & Murray BG (2001) A new Achyranthes (Amaranthaceae) from Phillip Island, Norfolk Island Group, South Pacific Ocean. New Zealand Journal of Botany 39, 1–8.
de Lange PJ & Murray BG (2003) Chromosome numbers of Norfolk Island endemic plants. Australian Journal of Botany 51, 211–215.
de Lange PJ, Gardner RO, Sykes WR, Crowcroft GM, Cameron EK, Stalker F, Christian ML, & Braggins JE (2005) Vascular flora of Norfolk Island: some additions and taxonomic notes. New Zealand Journal of Botany 43, 563–596.
de Lange PJ, Johnson PN, Norton DA, Hitchmough R, Heenan PB, Courteney SP, Molloy B.P.J, Ogle C.C & Rance BD (2004) Threatened and uncommon plants of New Zealand. New Zealand Journal of Botany 42, 45–76.
Debus SJS (2012) Norfolk Island Boobook chick deaths. Boobook 30, 6.
DEWHA (Department of the Environment, Water, Heritage and the Arts) (2008a) Approved Conservation Advice for Advena campbellii campbellii. Department of the Environment, Water, Heritage and the Arts, Canberra. Accessed 1 February 2024.
DEWHA (Department of the Environment, Water, Heritage and the Arts) (2008b). Approved Conservation Advice for Mathewsoconcha grayi ms (a snail). Department of the Environment, Water, Heritage and the Arts, Canberra. Accessed 1 February 2024.
DEWHA (Department of the Environment, Water, Heritage and the Arts) (2008c). Approved Conservation Advice for Mathewsoconcha phillipii (Phillip Island Helicarinoid Snail). Department of the Environment, Water, Heritage and the Arts, Canberra. Accessed 1 February 2024.
DEWHA (Department of the Environment, Water, Heritage and the Arts) (2008d). Approved Conservation Advice for Mathewsoconcha suteri (a snail). Department of the Environment, Water, Heritage and the Arts, Canberra. Accessed 1 February 2024.
DEWHA (Department of the Environment, Water, Heritage and the Arts) (2008e). Approved Conservation Advice for Quintalia stoddartii (Stoddart's Helicarionid Land Snail). Department of the Environment, Water, Heritage and the Arts, Canberra. Accessed 1 February 2024.
Di Fonzo MMI, Nicol S, Possingham HP, Flakus S, West JG, Failing L, Long G & Walshe T (2017) Cost‑Effective Resource Allocator: A decision support tool for threatened species management. Parks 23(1), 101–113.
Director of National Parks (2008) Norfolk Island National Park and Norfolk Island Botanic Garden Management Plan 2008-2018. Department of the Environment, Water, Heritage and the Arts, Canberra.
Director of National Parks (2010) Norfolk Island Region Threatened Species Recovery Plan. Department of the Environment, Water, Heritage and the Arts, Canberra.
Director of National Parks (2011) Norfolk Island National Park and Botanic Garden Climate Change Strategy 2011-2016. Department of Sustainability, Environment, Water, Population and Communities, Canberra.
Director of National Parks (2018) Temperate East Marine Parks Network Management Plan 2018. Director of National Parks, Canberra.
Director of National Parks (2020) Norfolk Island National Park and Norfolk Island Botanic Garden Management Plan 2020. Director of National Parks, Canberra.
Director of National Parks (2021) Norfolk Island Threatened Species Recovery Plan: Prioritising management activities to conserve threatened species. Unpublished report.
DoEE (Department of the Environment and Energy) (2017) Recovery team governance—Best practice guidelines. Commonwealth of Australia. Accessed 1 February 2024.
Doran GT (1981) There’s a SMART way to write management’s goals and objectives. Management review 70(11), 35–36.
DSEWPaC (Department of Sustainability, Environment, Water, Population and Communities) (2012a) Marine bioregional plan for the North Marine Region. Accessed 1 February 2024.
DSEWPaC (Department of Sustainability, Environment, Water, Population and Communities) (2012b) Marine bioregional plan for the North-west Marine Region. Accessed 1 February 2024.
DSEWPaC (Department of Sustainability, Environment, Water, Population and Communities) (2012c) Marine bioregional plan for the South-west Marine Region. Accessed 1 February 2024.
DSEWPaC (Department of Sustainability, Environment, Water, Population and Communities) (2012d) Marine bioregional plan for the Temperate East Marine Region. Accessed 1 February 2024.
Dunlop M & Brown PR (2008) Implications of Climate Change for Australia’s National Reserve System: A Preliminary Assessment. CSIRO Sustainable Ecosystems, report to the Department of Climate Change and the Department of the Environment, Water, Heritage and the Arts, Canberra.
Dutson G (2013) Population densities and conservation status of Norfolk Island forest birds. Bird Conservation International 23, 271–282.
Edwards ED (1985) Report on the Lepidoptera collected on Norfolk and Phillip Islands. Unpublished Report to the Australian National Parks and Wildlife Service, Canberra.
Elix JA & Streimann H (1985) Lichens and Bryophytes of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service, Canberra.
Elix JA, Streimann H & Archer AW (1992) The lichens of Norfolk Island 2: The genera Cladonia, Pertusaria, Pseudocyphellaria and Ramalina. Proceedings of the Linnean Society of New South Wales 113(1), 72–76.
Erwin T, Bathols J, Heady C, Bedin T, Wilson L, Narsey S, Rafter T, Bhend J & Clarke J (2015) CMIP5 Climatologies. v5. Unpublished analysis. CSIRO Data Access Portal. Accessed 30 January 2024.
ESSS (Endangered Species Scientific Subcommittee) (1995) Commonwealth Listing Advice on Incidental catch (or bycatch) of seabirds during oceanic longline fishing operations. Commonwealth of Australia.
Fernández-Palacios JM, Kreft H, Irl SDH, Norder S, Ah-Peng C & Borges PAV (2021) Scientists’ warning–The outstanding biodiversity of islands is in peril. Global ecology and conservation 31, e01847.
Fitzherbert K & Peter J (1988) Status and movement of Australian migratory birds Vol 1. Procellariiformes Part II. Royal Australasian Ornithologists Union, Melbourne.
Frankham R, Bradshaw CJ & Brook BW (2014) Genetics in conservation management: revised recommendations for the 50/500 rules, Red List criteria and population viability analyses. Biological Conservation 170, 56–63.
Fullagar PJ & Disney HJ (1975) The birds of Lord Howe Island: a report on the rare and endangered species. ICBP Bulletin 12, 187–202.
Fullagar PJ (1978) Norfolk Island birds. Unpublished report to RAOU Congress, Norfolk Island.
Gardner RO & de Lange PJ (2002) Revision of Pennantia (Icacinaceae), a small isolated genus. Journal of the Royal Society of New Zealand 32, 669–695.
Garnett ST & Baker GB (2021) The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne.
Garnett ST & Crowley GM (2000) The Action Plan for Australian Birds. Environment Australia.
Garnett ST, Szabo J & Dutson G (2011) The Action Plan for Australian Birds 2010. CSIRO Publishing, Melbourne.
Gautschi D, Heinsohn R, Crates R, Macgregor NA, Wilson M & Stojanovic D (2022) Utilization of modified and artificial nests by endemic and introduced parrots on Norfolk Island. Restoration Ecology 30(5), e13586.
Giachino PM (2005) Revision of the Australian Anillina (Coleoptera, Carabidae, Bembidiini) Results of the Zoological Missions to Australia of the Regional Museum of Natural Sciences of Turin. II. Monografie del Museo regionale di Scienze naturali, Torino 42, 137–238.
Gilmour PM & Helman CE (1989a) A Survey of Quality Plant Communities of Norfolk Island Outside the national park. Report to the Australian National Parks and Wildlife Service, Norfolk Island.
Gilmour PM & Helman CE (1989b) The Vegetation of Norfolk Island National Park. Report to the Australian National Parks and Wildlife Service, Norfolk Island.
Gorta S (2023) unpublished data.
Green P (1994) Introduction. Norfolk Island and Lord Howe Island, in A Orchard (ed), Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra. pp. 1–24.
Greene TC (2003) Breeding biology of red-crowned parakeets (Cyanoramphus novaezelandiae novaezelandiae) on Little Barrier Island, Hauraki Gulf, New Zealand. Notornis 50, 83–99.
Groeneveld KM (1989) Conservation biology of the endangered species Hibiscus insularis. Unpublished Report to the Australian National Parks and Wildlife Service.
Hall LS, Krausman PR & Morrison ML (1997) The habitat concept and a plea for standard terminology. Wildlife Society Bulletin 25, 173–182.
Halpin LR, Carlile N, Baker GB & Garnett ST (2021b) White-necked Petrel Pterodroma cervicalis cervicalis, in ST Garnett & GB Baker (eds), The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne. pp. 177–179.
Halpin LR, Mott R, Clay TA, Humphries GRW, Chatwin TA, Carlile N & Clarke RH (2022) Predicting the foraging habitats of sympatrically breeding gadfly petrels in the South Pacific Ocean. Frontiers in Marine Science 9, 853104.
Halpin LR, Terrington DI, Jones HP, Mott R, Wong WW, Dow DC, Carlile N & Clarke RH (2021a) Arthropod predation of vertebrates structures trophic dynamics in island ecosystems. The American Naturalist 198(4), 540–550.
Hay JR (1986) Bird Conservation in the Pacific Islands. Study Report 7. International Council for Bird Preservation, Cambridge.
Hayes RA, Crossland MR, Hagman M, Capon RJ & Shine R (2009) Ontogenetic variation in the chemical defenses of cane toads (Bufo marinus): toxin profiles and effects on predators. Journal of chemical ecology 35(4), 391–399.
Heinsohn R (2019) Review of the translocation of Norfolk Island Green Parrots from Norfolk Island to Phillip Island. Report to the Director of National Parks, Canberra.
Hermes N, Evans O & Evans B (1986) Norfolk Island birds: a review 1985. Notornis 33, 141–149.
Heterick BE & Shattuck S (2011) Revision of the ant genus Iridomyrmex (Hymenoptera: Formicidae). Zootaxa 2845, 1–174.
Hicks J & Greenwood D (1989) Rescuing Norfolk Island’s Parrot. Birds International 2, 35–47.
Hicks J & Preece M (1991) Green Parrot. 1991 Recovery Plan. Unpublished report to the Australian National Parks and Wildlife Service.
Hill R (2002) Recovery Plan for the Norfolk Island Green Parrot Cyanoramphus novaeseelandiae cookii. Environment Australia, Canberra.
Hoare M (1969) Norfolk Island—An Outline of its History 1774–1968. University of Queensland Press, St. Lucia, Queensland.
Hoffmann BD (2020) Argentine Ant Eradication Strategy Norfolk Island 2021-2026. CSIRO.
Holloway JD (1977) The Lepidoptera of Norfolk Island: their biogeography and ecology (Series Entomologica 13). W Junk publisher, The Hague.
Hoskin CJ (2011) The invasion and potential impact of the Asian House Gecko (Hemidactylus frenatus) in Australia. Austral Ecology 36(3), 240–251.
Hoye GA, Law BS & Allison FR (2008) East-coast Free-tailed Bat, in R Strahan & S Van Dyck (eds), The Mammals of Australia. 3rd ed. Reed New Holland, Sydney.
Hughes L (2003) Climate change and Australia: trends, projections, and impacts. Austral Ecology 28, 423–443.
Hyder Consulting (2008) The Impacts and Management Implications of Climate Change for the Australian Government’s Protected Areas. Report to the Department of Climate Change and the Department of the Environment, Water, Heritage and the Arts, Canberra.
Hyman I & Köhler F (2020) Report on survey of land snails on Norfolk Island. Australian Museum, Sydney.
Hyman I (2005) Taxonomy, systematic, and evolutionary trends in Helicarionida (Mollusca, Pulmonata). PhD Thesis, University of Sydney.
Hyman IT, Caiza J & Köhler F (2023) Systematic revision of the microcystid land snails endemic to Norfolk Island (Gastropoda: Stylommatophora) based on comparative morpho-anatomy and mitochondrial phylogenetics. Invertebrate Systematics 37(5–6), 334–443.
Invasive Species Compendium (2022a) Norfolk Island. Accessed 30 January 2024.
Invasive Species Compendium (2022b) Rattus rattus (black rat) Data Sheet. Accessed 30 January 2024.
Invasive Species Council & TierraMar (2021) The Native Plant Communities of Norfolk Island. Invasive Species Council, Katoomba, NSW.
Invasive Species Council and Island Conservation (2017) Norfolk Island: Protecting an Ocean Jewel. Recommendations for stronger biosecurity for the Norfolk Island Group. Invasive Species Council and Island Conservation, Fairfield, Victoria.
IPBES (2019) Global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. ES Brondizio, J Settele, S Díaz & HT Ngo (eds). IPBES secretariat, Bonn, Germany.
IPCC (2021) Climate Change 2021: The Physical Science Basis (Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change). V Masson-Delmotte, P Zhai, A Pirani, SL Connors, C Péan, S Berger, N Caud, Y Chen, L Goldfarb, MI Gomis, M Huang, K Leitzell, E Lonnoy, JB R Matthews, TK Maycock, T Waterfield, O Yelekçi, R Yu & B Zhou (eds). Cambridge University Press, Cambridge.
Iredale T (1910) Bird life on the Kermadec Islands. Emu. 10, 2–16.
Iredale T (1945) The land mollusca of Norfolk Island. Australian Zoologist 11, 46–71.
IUCN (2020) IUCN Redlist. Accessed 30 January 2024.
IUCN (2022) Version 2022-1. Accessed 21 December 2022.
Jean Rice Architect, Context Pty Ltd & GML Heritage Pty Ltd (2016) Kingston and Arthur’s Vale Historic Area Heritage Management Plan. Commonwealth of Australia.
Johns PM (1967) A note on the introduced millipedes of New Zealand. New Zealand Entomologist 3(5), 60–62.
Jones JG & McDougall I (1973) Geological history of Norfolk and Philip islands, southwest Pacific Ocean. Journal of the Geological Society of Australia 20(3), 239–254.
Kearney SG, Watson JE, Reside AE, Fisher DO, Maron M, Doherty TS, Legge SM, Woinarski JC, Garnett ST, Wintle BA & Ritchie EG (2023) Threat-abatement framework confirms habitat retention and invasive species management are critical to conserve Australia's threatened species. Biological Conservation 277, 109833.
Kirk DA, Park AC, Smith AC, Howes BJ, Prouse BK, Kyssa NG, Fairhurst EN & Prior KA (2018) Our use, misuse, and abandonment of a concept: Whither habitat? Ecology and Evolution 8(8), 4197–4208.
Koch LE (1984) A new species of Cormocephalus centipede (Chilopoda: Scolopendridae) from Phillip Island in the South Pacific. Journal of Natural History 18(4), 617–621.
Levick S & Johnson S (2023) Norfolk Island landcover mapping from airborne LiDAR. v2. Data collection. CSIRO Data Access Portal. Accessed 30 January 2024.
Lindsey TR (1986) The Seabirds of Australia: the national photographic index of Australian Wildlife. Angus and Robertson, North Ryde, NSW.
Lombal AJ, Wenner TJ, Carlile N, Austin JJ, Woehler E, Priddel D & Burridge CP (2016) Population genetic and behavioral variation of the two remaining colonies of Providence petrel (Pterodroma solandri). Conservation Genetics 18, 117–129.
Lowe S, Browne M, Boudjelas S & De Poorter M (2000) 100 of the world's worst invasive alien species: a selection from the global invasive species database (Vol. 12). Invasive Species Specialist Group, Auckland.
Macgregor NA, Wilson M, Brown SM, Goumas M, Heinsohn R, Clarke RH, Ortiz-Catedral L, Greenup N, Christian M, Greenwood D, Ward R & Garnett ST (2021) Norfolk Island Green Parrot Cyanoramphus novaezelandiae cookii, in ST Garnett & GB Baker (eds) The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne. pp. 432–435.
Major R (1989) Reproductive output and recruitment of the Norfolk Island Scarlet Robin (Petroica multicolor multicolor) Phase II. Report to the Australian National Parks and Wildlife Service, Canberra.
Martoni F, Achari S, Blacket MJ, Brohier ND, Constable F, Dugdale T, Ekanayake P, Kant P, Kelly G, Kinoti C, Li T, Lovelock D, Mann R, Nogarotto E, Sawbridge T, Smith R, Wainer J & Rodoni B (2023) Plant pest and disease survey on Norfolk Island. Department of Energy, Environment and Climate Change (Victoria), report to the Department of Infrastructure, Transport, Regional Development, Communications and the Arts, Canberra.
Maynard GV, Lepschi BJ & Malfroy SF (2018) Norfolk Island quarantine survey 2012–2014—a comprehensive assessment of an isolated subtropical island. Proceedings of the Linnean Society of New South Wales 140, 7–243.
McCormack RB & Coughran J (2009) Norfolk Island Freshwater Aquatic Survey 2009 (Internal Project Report, The Australian Crayfish Project). Australian Aquatic Biological Pty Ltd, Karua, NSW.
McJannet D, Marano J, Petheram C, Tavener N & Greenwood D (2023) Quantifying rainfall and cloud water interception in upland forests of Norfolk Island. Hydrological Processes 37(7), e14945.
Medway DG (2002) History and causes of the extirpation of the Providence petrel (Pterodrma solandri) on Norfolk Island. Notornis 49 (4), 246–258.
Melville R (1969) The endemics of Phillip Island. Biological Conservation 1(2), 170–172.
Merton DV (1970) Kermadec Island expedition reports: a general account of bird life. Notornis 17, 147–199.
Mills K (2007a) The Flora of Norfolk Island. 1. The Indigenous Flora. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2007b) The Flora of Norfolk Island. 8. Indigenous Plant Species Recorded in Public Reserves on Norfolk Island. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2007c) The Flora of Norfolk Island. 3. The Coastal Species. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2007d) The Flora of Norfolk Island. 5. Field Survey of the Norfolk Island Endemic Shrub Euphorbia norfolkiana (Euphorbiaceae). Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2007e) The Flora of Norfolk Island. 2. Epiphytes and Mistletoes. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2007f) The Flora of Norfolk Island. 7. Endemic and Threatened Species. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2009a) The Flora of Norfolk Island. 9. The Vegetation of Nepean Island (including Errata and Addenda for Papers 1 to 8). Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2009b) The Vegetation of Phillip Island, Norfolk Island Group. Envirofund 2007/2008. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2010) The Flora of Norfolk Island. 11. Field Survey and Assessment of the Critically Endangered Endemic Plant Wikstroemia australis (Kurrajong). Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2012a) The Flora of Norfolk Island. Report 16. The Wetland Plants. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2012b) The Flora of Norfolk Island. Report 14. The Endangered Plants in the national park: Field Survey and Review. Kevin Mills & Associates, Jamberoo, NSW.
Mills K (2017a) Survey of public reserves on Norfolk Island for threatened plant species: 3. Point Ross Reserve and Bumbora Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017b) Survey of public reserves on Norfolk Island for threatened plant species: 8. Ball Bay Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017c) Survey of public reserves on Norfolk Island for threatened plant species: 1. The Kingston Reserves. Prepared for Norfolk Island Regional Council.
Mills K (2017d) Survey of public reserves on Norfolk Island for threatened plant species: 6. Anson Bay Reserve and Selwyn Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017e) Survey of public reserves on Norfolk Island for threatened plant species: 7. Hundred Acres Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017f) Survey of public reserves on Norfolk Island for threatened plant species: 4. Cascade Reserve including Quarantine Reserve. Prepared for Norfolk Island Regional Council.
Mills K (2017g) Survey of public reserves on Norfolk Island for threatened plant species: 5. Two Chimneys Reserve. Prepared for Norfolk Island Regional Council.
Moore BP (1985) The Carabidae of Norfolk Island, in GE Ball (ed), Taxonomy, phylogeny and zoogeography of beetles and ants. W Junk publisher, Doordrecht, Netherlands. pp. 236–256.
Mosley JG (2001) Island on the Brink—A Conservation Strategy for Norfolk Island. Norfolk Island Conservation Society, Norfolk Island.
Mound LA & Wells A (2015) Endemics and adventives: Thysanoptera (Insecta) biodiversity of Norfolk, a tiny Pacific Island. Zootaxia 3964 (2), 183–210.
Munstermann MJ, Heim NA, McCauley DJ, Payne JL, Upham NS, Wang SC & Knope ML (2022) A global ecological signal of extinction risk in terrestrial vertebrates. Conservation Biology 36(3), e13852.
Nance AH, Mitchell WF, Clarke RH, Wilson M, Brown SM, Macgregor NA, Dutson G & Garnett ST (2021c) Slender-billed White-eye Zosterops tenuirostris, in ST Garnett & GB Baker (eds) The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne. pp. 763–765.
Nance AH, Mitchell WF, Clarke RH, Wilson M, Brown SM, Macgregor NA, Dutson G & Garnett ST (2021b) Norfolk Island Robin Petroica multicolor, in ST Garnett & GB Baker (eds) The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne. pp. 741–744.
Nance AH, Mitchell WF, Wilson M, Brown SM, Clarke RH, Macgregor NA, Ward R & Garnett ST (2021a) Norfolk Island Golden Whistler Pachycephala pectoralis xanthoprocta, in ST Garnett & GB Baker (eds) The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne. pp. 709–710.
Nance AH, Mitchell WF, Dawlings F, Cook CN & Clarke RH, (2023) Rodent predation and specialised avian habitat requirements drive extinction risk for endemic island songbirds in the south-west Pacific. Emu - Austral Ornithology 123(3), 217–231.
Naumann ID (1984) Review of Norfolk and Phillip Island Hymenoptera (excluding Formicidae). Unpublished report to the Australian National Parks and Wildlife Service.
Neuweger D, White P & Ponder WF (2001) Land snails from Norfolk Island sites. Records of the Australian Museum, Supplement 27, 115–122.
NIP&FS (Norfolk Island Parks and Forestry Service) (2003) Nepean Island Plan of Management Part B Section 8.
NIRC (Norfolk Island Regional Council) (2003) Norfolk Island Heritage Act 2002 Heritage Register. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2016) (as amended). Norfolk Island Plan 2002. Latest amendment approved 21 March 2016. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2016) Norfolk Island Community Strategic Plan 2016–2026: Our Plan for the Future. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2017) Norfolk Island Heritage and Culture Strategy 2017–2020. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2018b) Norfolk Island Regional Council Asset Management Policy. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2018a) The Norfolk Island Environment Strategy 2018-2023. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2020a) Plan of Management Anson Bay Reserve. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2020b) Plan of Management Ball Bay Reserve. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2020c) Plan of Management Bumbora Reserve. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2020d) Plan of Management Cascade Reserve. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2020e) Plan of Management Cemetery Reserve. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2020f) Plan of Management Government House Reserve. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2020g) Plan of Management Headstone Reserve. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2020h) Plan of Management Hundred Acres Reserve. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2020i) Plan of Management Kingston Common Reserve. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2020j) Plan of Management Kingston Recreation Reserve. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2020k) Plan of Management Middleridge Reserve. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2020l) Plan of Management Nepean Reserve. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2020m) Plan of Management Point Hunter Reserve. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2020n) Plan of Management Point Ross Reserve. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2020o) Plan of Management Selwyn Reserve. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2020p) Plan of Management Stock Reserve. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2020q) Plan of Management Two Chimneys Reserve. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2020r) Plan of Management War Memorial Reserve. Norfolk Island Regional Council, Norfolk Island.
NIRC (Norfolk Island Regional Council) (2021) Norfolk Island Regional Council Pest Management Plan (NIRCPMP) 2021-2026. Norfolk Island Regional Council, Norfolk Island.
Norfolk Island Conservation Society (1988) A conservation strategy for Norfolk Island. Unpublished report, Norfolk Island Conservation Society.
O'Dowd DJ, Green PT & Lake PS (2003) Invasional ‘meltdown’ on an oceanic island. Ecology Letters 6(9), 812–817.
O’Dwyer T, Carlile N, O’Neill L & Halpin LR (2023) Changing fortunes of the Black-winged Petrel Pterodroma nigripennis following the Lord Howe Island Rodent Eradication Project - interactions with other recovering species. Bird Conservation International 33: e18.
Oliver EC, Burrows MT, Donat MG, Sen Gupta A, Alexander LV, Perkins-Kirkpatrick SE, Benthuysen JA, Hobday AJ, Holbrook NJ, Moore PJ, Thomsen MS, Wernberg T & Smale TA (2019) Projected marine heatwaves in the 21st century and the potential for ecological impact. Frontiers in Marine Science 6, 734.
Olsen P (1996) Re-establishment of an endangered subspecies: the Norfolk Island Boobook. Bird Conservation International 6, 63–70.
Olsen P (1997) Recovery Plan for the Norfolk Island Boobook Owl Ninox novaeseelandiae undulata. Environment Australia, Canberra.
Olsen PD (1986) Status and conservation of the Norfolk Island Boobook Owl Ninox novaeseelandiae undulata. Unpublished report to the Australian National Parks and Wildlife Service.
Olsen PD, Mooney NJ & Olsen J (1989) Status and conservation of the Norfolk Island Boobook Ninox novaeseelandiae undulata, in BU Meyburg & RD Chancellor (eds) Raptors in the Modern World. WWGBP, Berlin. pp. 123–129.
O'Neill L (2006) The breeding and feeding ecology of the Sooty Tern Sterna fuscata on Lord Howe Island. PhD Thesis, Charles Sturt University, Albury, NSW.
Orchard A (ed) (1994) Flora of Australia. Vol. 49. Oceanic Islands 1. Australian Government Publishing Service, Canberra.
Ortiz-Catedral L (2013) The Population and Status of Green Parrot (Tasman Parakeet) Cyanoramphus cookii on Norfolk Island. Unpublished report to the Director of National Parks.
Ortiz-Catedral L, Kearvell JC, Hauber ME & Brunton DH (2009) Breeding biology of the critically endangered Malherbe’s parakeet on Maud Island, New Zealand, following the release of captive‑bred individuals. Australian Journal of Zoology 57, 433–439.
Ortiz-Catedral L, Nias R, Fitzsimons J, Vine S & Christian M (2018) Back from the brink–again: the decline and recovery of the Norfolk Island green parrot, in S Garnett, P Latch, D Lindenmayer & J Woinarski (eds) Recovering Australian Threatened Species: A Book of Hope. CSIRO Publishing, Australia.
Petheram C, Taylor A, Hughes J, Philip S, Tavener N, Greenwood D, Taylor N, Wilson PR, Raiber M, Turnadge C, Yang A, Seo L, Davies P, Vanderzalm J, Davis A, Crane P, Gallant J, Ahmed W, Rogers L, Ticehurst C, Marvanek S, Christoph G, Bui E, Grice T, Crosbie R, Metcalfe S, Fitzpatrick R, Schepen A, Levick S, Nobbs C, Cahill K & Vaze J (2020) Norfolk Island Water Resource Assessment. A report to the Australian Government from the CSIRO Norfolk Island Water Resource Assessment team. CSIRO, Australia.
PIER (Pacific Islands at Risk) (2002) Schinus terebinthifolius Risk Assessment. Accessed 30 January 2024.
Pierce R (2002) Pacific rats: their impact on two small seabird species in the Hen and Chicken Islands, New Zealand, in CR Veitch & MN Clout (eds) Turning the tide: the eradication of invasive species. Occasional Paper of the IUCN Species Survival Commission No. 27. IUCN, Gland, Switzerland.
Ponder WF (1997) Conservation status, threats and habitat requirements of Australian terrestrial and freshwater mollusca. Memoirs of the Museum of Victoria 56, 421–430.
Priddel D, Carlile N, Evans O, Evans B & McCoy H (2010) A review of the seabirds of Phillip Island in the Norfolk Island Group. Notornis 57, 113–127.
Rasheed AA, Hambley K, Chan G, de la Rosa CA, Larison B & Blumstein DT (2018) Persistence of antipredator behavior in an island population of California quail. Ethology 124(3),155–160.
Reid AM, Smith K and Beatson M (2018) Revision of the genus Lamprima Latreille, 1804 (Coleoptera: Lucanidae). Zootaxa 4446, 151–202.
Renner MAM & Beadel SM (2011) Taeniophyllum norfolkianum: a second genus of Vandeae (Orchidaceae) indigenous to New Zealand. New Zealand Journal of Botany 49(3), 435–439.
Rentz DDF (1988) The orthopteroid insects of Norfolk Island with descriptions of some related species from Lord Howe Island, South Pacific. Invertebrate Systematics 2, 1013–1077.
Robinson D (1988) Ecology and Management of the Scarlet Robin, White-breasted White-eye, and Long-billed White-eye of Norfolk Island. Consultants’ report to the Australian National Parks and Wildlife Service, Canberra.
Robinson D (1997) An evaluation of the status of the Norfolk Island Robin following rat-control and weed-control works in the Norfolk Island National Park. Report to Environment Australia, Canberra.
Rodda G, Fritts T, Campbell EW III, Dean-Bradley K, Perry G & Qualls C (2002) Practical concerns in the eradication of island snakes, in CR Veitch & MN Clout (eds), Turning the tide: the eradication of invasive species. Occasional Paper of the IUCN Species Survival Commission No. 27. IUCN, Gland, Switzerland. pp. 260–265.
Schodde R, Fullagar P & Hermes N (1983) A review of Norfolk Island birds past and present (Special Publication No. 8). Australian National Parks and Wildlife Service, Canberra.
Shine R (2010) The ecological impact of invasive cane toads (Bufo marinus) in Australia. The Quarterly Review of Biology 85(3), 253–291.
Simmonds SA (2019) Habitat use by Tasman Parakeets (Cyanoramphus cookii) and Crimson Rosellas (Platycercus elegans) on Norfolk Island, South Pacific. MSc Thesis, Massey University, Auckland.
Skirrow MJ (2019) Estimating the population size of two critically endangered South Pacific parakeets: the Tasman Parakeet and Malherbe's Parakeet. MSc Thesis, Massey University, Auckland.
Smith BJ (1992) Non-marine Mollusca, in WWK Houston (ed) Zoological Catalogue of Australia Volume 8. Australian Government Publishing Service, Canberra.
Smith I, Clark G & White P (2001) Mammalian and reptilian fauna from Emily and Cemetery Bays Norfolk Island. Records of the Australian Museum, Supplement 27, 75–79.
Smithers CN & Disney HJ (1969) The distribution of terrestrial and freshwater birds on Norfolk Island. Australian Zoologist 15, 127–140.
Smithers CN (1998) A species list and bibliography of the insects recorded from Norfolk Island. Technical Reports of the Australian Museum 13, 1–55.
Smithers CN, Peters JV & Thornton IWB (1999) The Psocoptera (Insecta) of Norfolk and Philip Islands: occurrence, status, and zoogeography. Proceedings of the Linnean Society of New South Wales 121, 101–111.
Sperring F, Webster W, Isaac B, Clarke R, Gautschi D, Heinsohn R, Olsen P, Weeks A, Macgregor N, Wilson M & Greenup N (2021b) Ecology, genetics, and conservation management of the Norfolk Island morepork and green parrot. Interim report to the NESP Threatened Species Recovery Hub, Brisbane.
Sperring VF, Brown SM, Macgregor NA, Olsen P, Clarke RH, Wilson M, Greenup N, Weeks A, Ward R, Greenwood D, Christian M & Garnett ST (2021a) Norfolk Island Morepork Ninox novaeseelandiae undulata, in ST Garnett & GB Baker (eds), The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne. pp. 360–363.
Standards Reference Group SERA (2017) National Standards for the Practice of Ecological Restoration in Australia. Second Edition. Society for Ecological Restoration Australasia. Accessed 30 January 2024.
Starr F, Starr K & Loope L (2003) Olea europaea subsp. cuspidata African olive. Report by Biological Sciences Resources Division, United States Geological Survey, Maui, Hawaii.
Stephens CG & Hutton JT (1954) A soil and land-use study of the Australian Territory of Norfolk Island, South Pacific Ocean. CSIRO, Melbourne.
Suarez AV, Holway DA & Case TJ (2001) Patterns of spread in biological invasions dominated by long-distance jump dispersal: insights from Argentine ants. Proceedings of the National Academy of Sciences 98 (3), 1095–1100.
Sykes W & Atkinson I (1988) Rare and Endangered Plants of Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service.
Tarburton MK (1981) Seabirds nesting on Norfolk Island. Notornis 28, 209–211.
Taylor RW & Brown DR (1985) Hymenoptera: Formicoidea, in DW Walton (ed), Zoological Catalogue of Australia Volume 2. Australian Government Publishing Service, Canberra.
Tennyson AJD, Taylor GA & Scofield RP (1989) Another visit to Macauley Island. Ornithological Society of New Zealand News 52, 4–5. Supplement to Notornis 36.
Tershy BR, Shen KW, Newton KM, Holmes ND & Croll DA (2015) The importance of islands for the protection of biological and linguistic diversity. BioScience 65(6), 592–597.
Tierney JW (1989) Report on investigation into kurrajong (Wikstroemia australis) decline and Phellinus noxius root rot control on Norfolk Island. Unpublished report to the Australian National Parks and Wildlife Service.
TSSC (Threatened Species Scientific Committee) (2003a) Listing Advice—11 Critically Endangered Norfolk Island Flora Species. Accessed 30 January 2024.
TSSC (Threatened Species Scientific Committee) (2003b) Listing Advice—15 Vulnerable Norfolk Island Flora Species. Accessed 30 January 2024.
TSSC (Threatened Species Scientific Committee) (2003c) Listing Advice—16 Endangered Norfolk Island Flora Species. Accessed 30 January 2024.
TSSC (Threatened Species Scientific Committee) (2003d) Listing Advice—Critically Endangered Achyranthes arborescens (Chaff Tree, Soft-wood). Accessed 29 January 2024.
TSSC (Threatened Species Scientific Committee) (2003e) Listing Advice—Critically Endangered Elatostema montanum (Mountain Procris, a herb). Accessed 29 January 2024.
TSSC (Threatened Species Scientific Committee) (2003f) Listing Advice—Critically Endangered Meryta latifolia (Shade Tree). Accessed 29 January 2024.
TSSC (Threatened Species Scientific Committee) (2003g) Listing Advice—Critically Endangered Wikstroemia australis (Kurrajong). Accessed 29 January 2024.
TSSC (Threatened Species Scientific Committee) (2003h) Commonwealth Listing Advice for Injury and fatality to vertebrate marine life caused by ingestion of, or entanglement in, harmful marine debris. Commonwealth of Australia
TSSC (Threatened Species Scientific Committee) (2009a) Commonwealth Listing Advice on Advena campbellii campbellii. Department of the Environment, Water, Heritage and the Arts. Accessed 29 January 2024.
TSSC (Threatened Species Scientific Committee) (2009b) Commonwealth Listing Advice on Mathewsoconcha grayi ms. Department of the Environment, Water, Heritage and the Arts, Canberra.
TSSC (Threatened Species Scientific Committee) (2009c) Commonwealth Listing Advice on Mathewsoconcha phillipii. Department of the Environment, Water, Heritage and the Arts, Canberra.
TSSC (Threatened Species Scientific Committee) (2009d) Commonwealth Listing Advice on Mathewsoconcha suteri. Department of the Environment, Water, Heritage and the Arts, Canberra.
TSSC (Threatened Species Scientific Committee) (2009e) Commonwealth Listing Advice on Quintalia stoddartii. Department of the Environment, Water, Heritage and the Arts, Canberra.
TSSC (Threatened Species Scientific Committee) (2016a) Conservation Advice Cyanoramphus cookii Norfolk Island green parrot. Department of the Environment, Canberra.
TSSC (Threatened Species Scientific Committee) (2016b). Conservation Advice Ninox novaeseelandiae undulata Norfolk Island boobook owl. Department of the Environment, Canberra.
TSSC (Threatened Species Scientific Committee) (2016c) Conservation Advice Streblus pendulinus Siah’s backbone. Department of the Environment, Canberra.
Turner JS, Smithers CN & Hoogland RD (1968) The Conservation of Norfolk Island. Australian Conservation Foundation, Melbourne.
Tweed J (2023) Phillip Island Survey March 2023. Unpublished data.
Varman RVJ (2015) Norfolk Island Snail Species Collections made between January and March 2015. Unpublished report.
Varman RVJ (2016) Norfolk Island Snail Species Collections made between January and March 2016. Unpublished report.
Varman RVJP (1991) Conchological Survey 1983-90: Manuscript of Land Mollusca Fossiliferous and Present Day. Unpublished manuscript.
Waldmann A (2016) Foraging ecology of the world's only population of the critically endangered Tasman parakeet (Cyanoramphus cookii) on Norfolk Island. MSc Thesis, Massey University, Auckland.
Watkins Consulting (1999) Australian Coastal Vulnerability Assessment Case Studies: Norfolk Island. Climate Change Program, Commonwealth Coastal Action Plan. Report prepared for Environment Australia and The Administration of Norfolk Island.
Weir TA (1985) Coleoptera. Report to Australian National Parks and Wildlife Service, Canberra.
Wilson L (2002) Norfolk Island rat and cat control evaluation. New Zealand Department of Conservation. Unpublished report to Environment Australia.
Wilson M (2016) Owl Survey Report, December 2016. Director of National Parks, Canberra.
Yong C, Ward M, Watson JEM, Reside AE, van Leeuwen S, Legge S, Geary WL, Lintermans M, Kennard MJ, Stuart S & Carwardine J (2023) The costs of managing key threats to Australia’s biodiversity. Journal of Applied Ecology 60, 898–910.
Ziesing P (1997) Norfolk Island Weed Control Manual. Environment Australia, Norfolk Island.
Zimmer H, Clements M, Cooper E, Jones D, Makinson R, Nargar K & Stevenson K (2023) Collateral damage: epiphytic orchids at risk from myrtle rust. Australian journal of botany 71, 523–536.
Other sources
Carlile N (2024) Personal communication by email, 12 January 2024, NSW Department of Planning and Environment.
Christian M (2024) Personal communication by email, 12 January 2024, independent researcher.
Christian J (2024) Personal communication by email, 11 January 2024, Parks Australia (Norfolk Island National Park).
Gautschi D (2024) Personal communication by email, 12 January 2024, Australian National University.
Gorta S (2024) Personal communication by email, 11 January 2024, University of New South Wales.
Mills K (2024) Personal communication by email, 11 January 2024, plant ecologist.
Ortiz-Catedral L (2024) Personal communication by email, 11 January 2024, World Parrot Trust & New Zealand Parrot Trust.
Tweed J (2024) Personal communication by email, 17 January 2024, University of Queensland.
Ward R (2024) Personal communication by email, 11 January 2024, Norfolk Island.
Wilson M (2024) Personal communication by email, 12 January 2024, Parks Australia (Norfolk Island National Park).